
A solution at pH 6 contains _________ H+ than the same amount of a solution at pH 8.
a. 20 times more
b. 100 times more
c. 2 times less
d. 100 times less
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
pH Scale
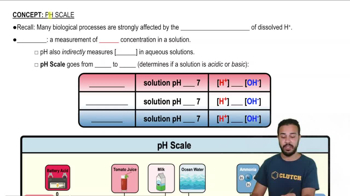
Hydrogen Ion Concentration

Logarithmic Scale

Changing the _________ would change it into an atom of a different element.
a. Number of electrons surrounding the nucleus of an atom
b. Number of protons in the nucleus of an atom
c. Electrical charge of an atom
d. Number of neutrons in the nucleus of an atom
What is chemically nonsensical about this structure?
Most of the unique properties of water result from the fact that water molecules
a. Are the most abundant molecules on Earth's surface.
b. Are held together by covalent bonds.
c. Are constantly in motion.
d. Are polar and form hydrogen bonds.
A can of cola consists mostly of sugar dissolved in water, with some carbon dioxide gas that makes it fizzy and makes the pH less than 7. In chemical terms, you could say that cola is an aqueous solution where water is the _________, sugar is a _________, and carbon dioxide makes the solution _________.
a. solvent . . . solute . . . basic
b. solute . . . solvent . . . basic
c. solvent . . . solute . . . acidic
d. solute . . . solvent . . . acidic
The atomic number of sulfur (S) is 16. Sulfur combines with hydrogen by covalent bonding to form a compound, hydrogen sulfide. Based on the number of valence electrons in a sulfur atom, predict the molecular formula of the compound. (Explain your answer.)
a. HS
b. H₂S
c. H₄S₂
d. H₄S
