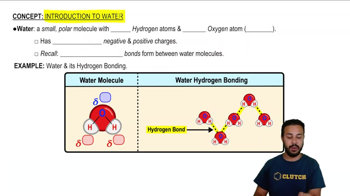
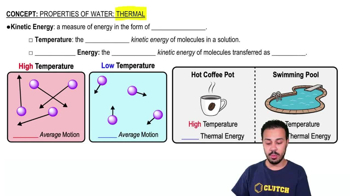
Changing the _________ would change it into an atom of a different element.
a. Number of electrons surrounding the nucleus of an atom
b. Number of protons in the nucleus of an atom
c. Electrical charge of an atom
d. Number of neutrons in the nucleus of an atom