An automobile tire has a maximum rating of 38.0 psi (gauge pressure). The tire is inflated (while cold) to a volume of 11.8 L and a gauge pressure of 36.0 psi at a temperature of 12.0 °C. On a hot day, the tire warms to 65.0 °C, and its volume expands to 12.2 L. Does the pressure in the tire exceed its maximum rating? (Note: The gauge pressure is the difference between the total pressure and atmospheric pressure. In this case, assume that atmospheric pressure is 14.7 psi.)
Ch.5 - Gases

Chapter 5, Problem 46
A 1.0-L container of liquid nitrogen is kept in a closet measuring 1.0 m by 1.0 m by 2.0 m. Assuming that the container is completely full, that the temperature is 25.0 °C, and that the atmospheric pressure is 1.0 atm, calculate the percent (by volume) of air that is displaced if all of the liquid nitrogen evaporates. (Liquid nitrogen has a density of 0.807 g/mL.)
 Verified step by step guidance
Verified step by step guidance1
Calculate the mass of liquid nitrogen using its density and the volume of the container: \( \text{mass} = \text{density} \times \text{volume} \).
Convert the mass of liquid nitrogen to moles using the molar mass of nitrogen (N2), which is approximately 28.02 g/mol.
Use the ideal gas law \( PV = nRT \) to find the volume of nitrogen gas at 25.0 °C and 1.0 atm. Remember to convert the temperature to Kelvin by adding 273.15.
Calculate the volume of the closet in liters: \( \text{volume} = \text{length} \times \text{width} \times \text{height} \).
Determine the percent of air displaced by dividing the volume of nitrogen gas by the volume of the closet and multiplying by 100 to get the percentage.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of a gas through the equation PV = nRT. This law is essential for understanding how gases behave under varying conditions and is crucial for calculating the volume of nitrogen gas produced when liquid nitrogen evaporates.
Recommended video:
Guided course

Ideal Gas Law Formula
Density and Volume Conversion
Density is defined as mass per unit volume (density = mass/volume). In this problem, knowing the density of liquid nitrogen allows us to convert its mass into volume, which is necessary to determine how much nitrogen gas will occupy the space when it evaporates.
Recommended video:
Guided course
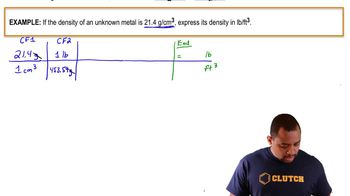
Density Conversion Example
Displacement of Air
When a substance is placed in a container, it displaces an equivalent volume of the fluid (or gas) that was previously in that space. In this scenario, calculating the volume of nitrogen gas produced will help determine the volume of air displaced in the closet, which is essential for finding the percent of air displaced.
Recommended video:
Guided course

Single Displacement Reactions
Related Practice
Textbook Question
1711
views
Textbook Question
A weather balloon is inflated to a volume of 28.5 L at a pressure of 748 mmHg and a temperature of 28.0 °C. The balloon rises in the atmosphere to an altitude of approximately 25,000 ft, where the pressure is 385 mmHg and the temperature is -15.0 °C. Assuming the balloon can freely expand, calculate the volume of the balloon at this altitude.
6405
views
2
rank
Textbook Question
A piece of dry ice (solid carbon dioxide) with a mass of 28.8 g sublimes (converts from solid to gas) into a large balloon. Assuming that all of the carbon dioxide ends up in the balloon, what is the volume of the balloon at 22 °C and a pressure of 742 mmHg?
4491
views
2
rank
Textbook Question
Which gas sample has the greatest pressure? Assume that all the samples are at the same temperature. Explain.
1332
views
