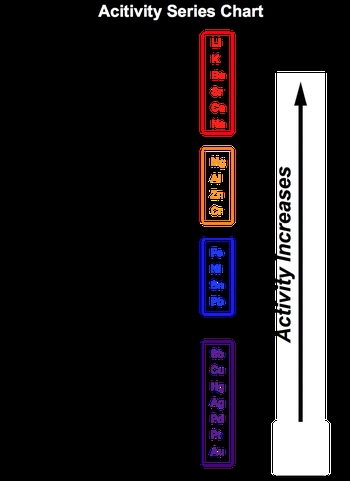
In chemistry, a single displacement reaction occurs when one element replaces another element in a compound. This can be illustrated with a simple example: if element A attempts to replace element B in the compound BC, it effectively removes B, resulting in a new compound AC, while B is left as a free element. However, not all elements can replace others; the ability to do so is determined by the activity series chart.
The activity series is a list that ranks elements based on their reactivity. An element positioned higher in the activity series can displace an element that is lower in the series. This means that if element A is above element B in the activity series, A can successfully replace B in the compound. Understanding this concept is crucial for predicting the outcomes of single displacement reactions and determining which reactions will occur.
In summary, single displacement reactions involve the replacement of one element in a compound by another, and the activity series chart is an essential tool for identifying which elements can participate in these reactions.