Textbook Question
Which compound would you expect to have greater surface tension: acetone [(CH3)2CO] or water (H2O)? Explain.
1822
views

 Verified step by step guidance
Verified step by step guidance
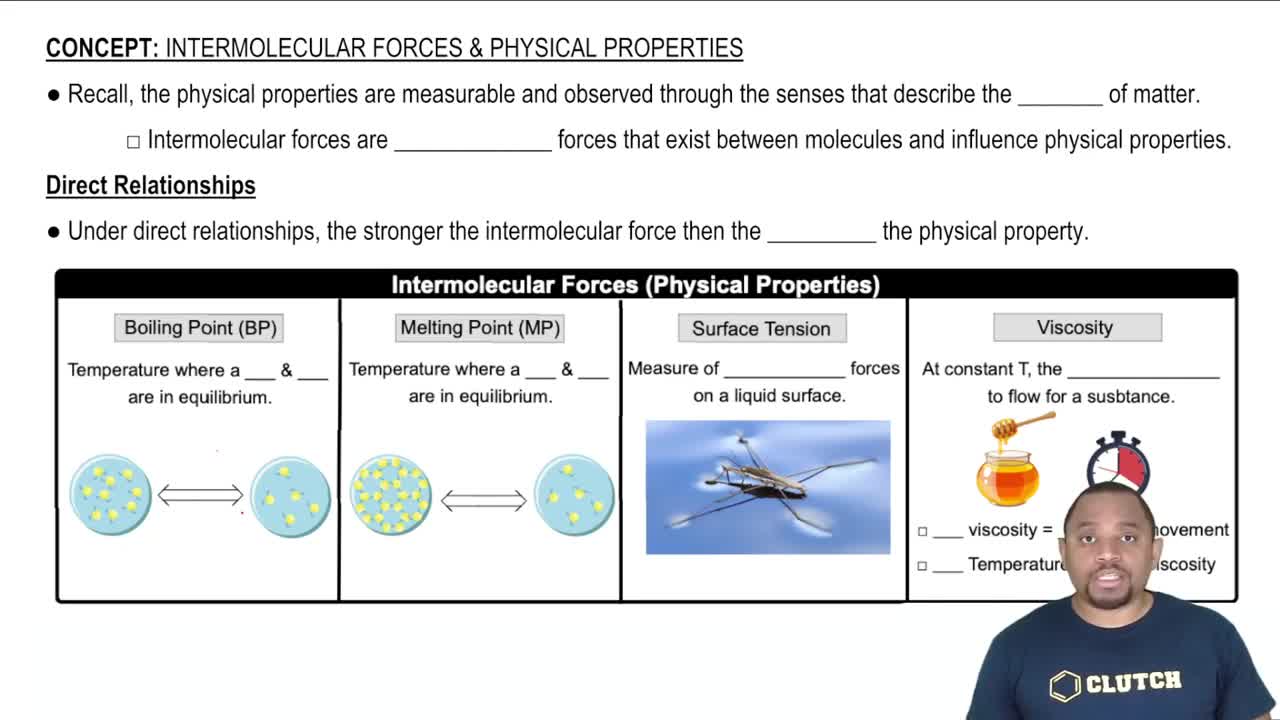

Which compound would you expect to have greater surface tension: acetone [(CH3)2CO] or water (H2O)? Explain.
The structures of two isomers of heptane are shown. Which of these two compounds would you expect to have the greater viscosity?
Determine whether each molecule is polar or nonpolar. c. BrCl5
When a thin glass tube is put into water, the water rises 1.4 cm. When the same tube is put into hexane, the hexane rises only 0.4 cm. Explain.