Textbook Question
Determine the pH of an HF solution of each concentration. In which cases can you not make the simplifying assumption that x is small? (Ka for HF is 6.8×10–4.) a. 0.250 M b. 0.0500 M c. 0.0250 M
2075
views
1
comments

 Verified step by step guidance
Verified step by step guidance

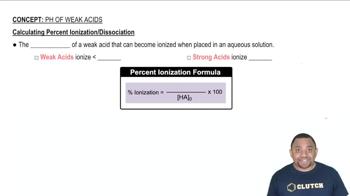

Determine the pH of an HF solution of each concentration. In which cases can you not make the simplifying assumption that x is small? (Ka for HF is 6.8×10–4.) a. 0.250 M b. 0.0500 M c. 0.0250 M
Determine the percent ionization of a 0.125 M HCN solution.
Determine the percent ionization of a 0.225 M solution of benzoic acid.