Textbook Question
The pH of a 1.00 M solution of urea, a weak organic base, is 7.050. Calculate the Ka of protonated urea.
1742
views

 Verified step by step guidance
Verified step by step guidance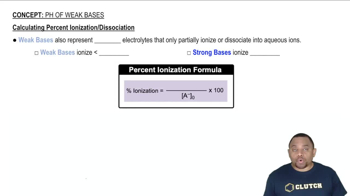


The pH of a 1.00 M solution of urea, a weak organic base, is 7.050. Calculate the Ka of protonated urea.
Lactic acid is a weak acid found in milk. Its calcium salt is a source of calcium for growing animals. A saturated solution of this salt, which we can represent as Ca(Lact)2, has a [Ca2+] = 0.26 M and a pH = 8.78. Assuming the salt is completely dissociated, calculate the Ka of lactic acid.