Textbook Question
Write equations for the half-reactions that occur at the anode and cathode for the electrolysis of each aqueous solution. b. KCl(aq) c. CuBr2(aq)

 Verified step by step guidance
Verified step by step guidance
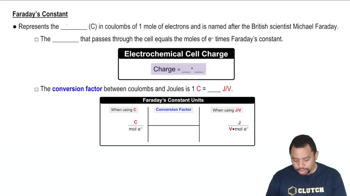

Write equations for the half-reactions that occur at the anode and cathode for the electrolysis of each aqueous solution. b. KCl(aq) c. CuBr2(aq)
Make a sketch of an electrolysis cell that electroplates copper onto other metal surfaces. Label the anode and the cathode and indicate the reactions that occur at each.
Silver can be electroplated at the cathode of an electrolysis cell by the half-reaction: Ag+(aq) + e– → Ag(s) What mass of silver would plate onto the cathode if a current of 6.8 A flowed through the cell for 72 min?
A major source of sodium metal is the electrolysis of molten sodium chloride. What magnitude of current produces 1.0 kg of sodium metal in 1 hour?