
The restriction enzymes XhoI and SalI cut their specific sequences as shown below:
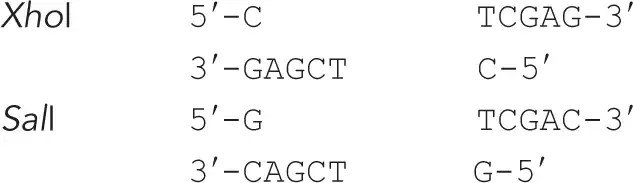
Can the sticky ends created by XhoI and SalI sites be ligated? If yes, can the resulting sequences be cleaved by either XhoI or SalI?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Restriction Enzymes

Sticky Ends
Ligation and Cleavage

The bacteriophage lambda genome can exist in either a linear form or a circular form.
How many fragments will be formed by restriction enzyme digestion with XhoI alone, with XbaI alone, and with both XhoI and XbaI in the linear and circular forms of the lambda genome?
The bacteriophage lambda genome can exist in either a linear form or a circular form.
Diagram the resulting fragments as they would appear on an agarose gel after electrophoresis.
The bacteriophage ϕX174 has a single-stranded DNA genome of 5386 bases. During DNA replication, double-stranded forms of the genome are generated. In an effort to create a restriction map of ϕX174, you digest the z-stranded form of the genome with several restriction enzymes and obtain the following results. Draw a map of the ϕX174 genome.
To further analyze the CRABS CLAW gene, you create a map of the genomic clone. The 11-kb EcoRI fragment is ligated into the EcoRI site of the MCS of the vector shown in Problem 18. You digest the double-stranded form of the genome with several restriction enzymes and obtain the following results. Draw, as far as possible, a map of the genomic clone of CRABS CLAW.
What restriction digest would help resolve any ambiguity in the map?
You have isolated a genomic clone with an EcoRI fragment of 11 kb that encompasses the CRABS CLAW gene. You digest the genomic clone with HindIII and note that the 11-kb EcoRI fragment is split into three fragments of 9 kb, 1.5 kb, and 0.5 kb.
Does this tell you anything about where the CRABS CLAW gene is located within the 11-kb genomic clone?
