DNA footprint protection is a method that determines whether proteins bind to a specific sample of DNA and thus protect part of the DNA from random enzymatic cleavage by DNase I. A 400-bp segment of cloned DNA is thought to contain a promoter. The cloned DNA is analyzed by DNA footprinting to help determine if it has the capacity to act as a promoter sequence. The accompanying gel has two lanes, each containing the cloned 400-bp DNA fragment treated with DNase I to randomly cleave unprotected DNA. Lane 1 is cloned DNA that was mixed with RNA polymerase II and several TFII transcription factors before exposure to DNase I. Lane 2 contains cloned DNA that was exposed only to DNase I. RNA pol II and TFIIs were not mixed with that DNA before adding DNase I. Approximately what length is the DNA region protected by RNA pol II and TFIIs?

 Sanders 3rd Edition
Sanders 3rd Edition Ch. 8 - Molecular Biology of Transcription and RNA Processing
Ch. 8 - Molecular Biology of Transcription and RNA Processing Problem 28a
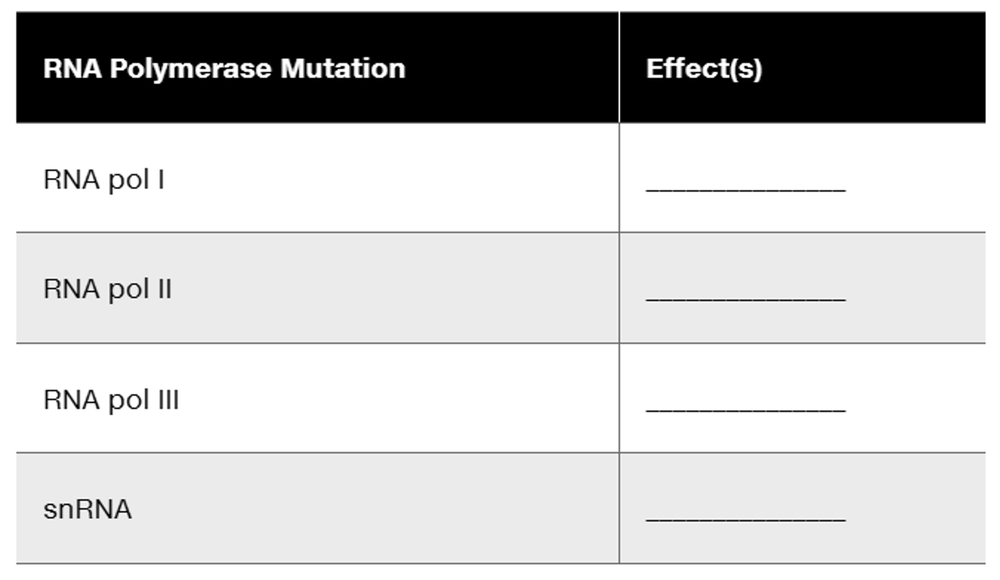
Problem 28aAssume that a mutation affects the gene for each of the following eukaryotic RNA polymerases. Match each mutation with the possible effects from the list provided. More than one effect is possible for each mutation.
Pre-mRNA does not have introns removed.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Eukaryotic RNA Polymerases
Mutations and Their Effects

Pre-mRNA Processing

DNA footprint protection is a method that determines whether proteins bind to a specific sample of DNA and thus protect part of the DNA from random enzymatic cleavage by DNase I. A 400-bp segment of cloned DNA is thought to contain a promoter. The cloned DNA is analyzed by DNA footprinting to help determine if it has the capacity to act as a promoter sequence. The accompanying gel has two lanes, each containing the cloned 400-bp DNA fragment treated with DNase I to randomly cleave unprotected DNA. Lane 1 is cloned DNA that was mixed with RNA polymerase II and several TFII transcription factors before exposure to DNase I. Lane 2 contains cloned DNA that was exposed only to DNase I. RNA pol II and TFIIs were not mixed with that DNA before adding DNase I. What additional genetic experiments would you suggest to verify that this region of cloned DNA contains a functional promoter?
Suppose you have a 1-kb segment of cloned DNA that is suspected to contain a eukaryotic promoter, including a TATA box, a CAAT box, and an upstream GC-rich sequence. The clone also contains a gene whose transcript is readily detectable. Your laboratory supervisor asks you to outline an experiment that will (1) determine if eukaryotic transcription factors (TF) bind to the fragment and, if so, (2) identify where on the fragment the transcription factors bind. All necessary reagents, equipment, and experimental know-how are available in the laboratory. Your assignment is to propose techniques to be used to address the two items your supervisor has listed and to describe the kind of results that would indicate binding of TF to the DNA and the location of the binding.
Assume that a mutation affects the gene for each of the following eukaryotic RNA polymerases. Match each mutation with the possible effects from the list provided. More than one effect is possible for each mutation.
Some pre-mRNA is not synthesized.
Assume that a mutation affects the gene for each of the following eukaryotic RNA polymerases. Match each mutation with the possible effects from the list provided. More than one effect is possible for each mutation.
Some rRNA is not synthesized.
Assume that a mutation affects the gene for each of the following eukaryotic RNA polymerases. Match each mutation with the possible effects from the list provided. More than one effect is possible for each mutation.
Some tRNA is not synthesized.
