Two curves are shown in the following energy diagram:
b. Which curve represents the spontaneous reaction, and which the nonspontaneous?

 Verified step by step guidance
Verified step by step guidance

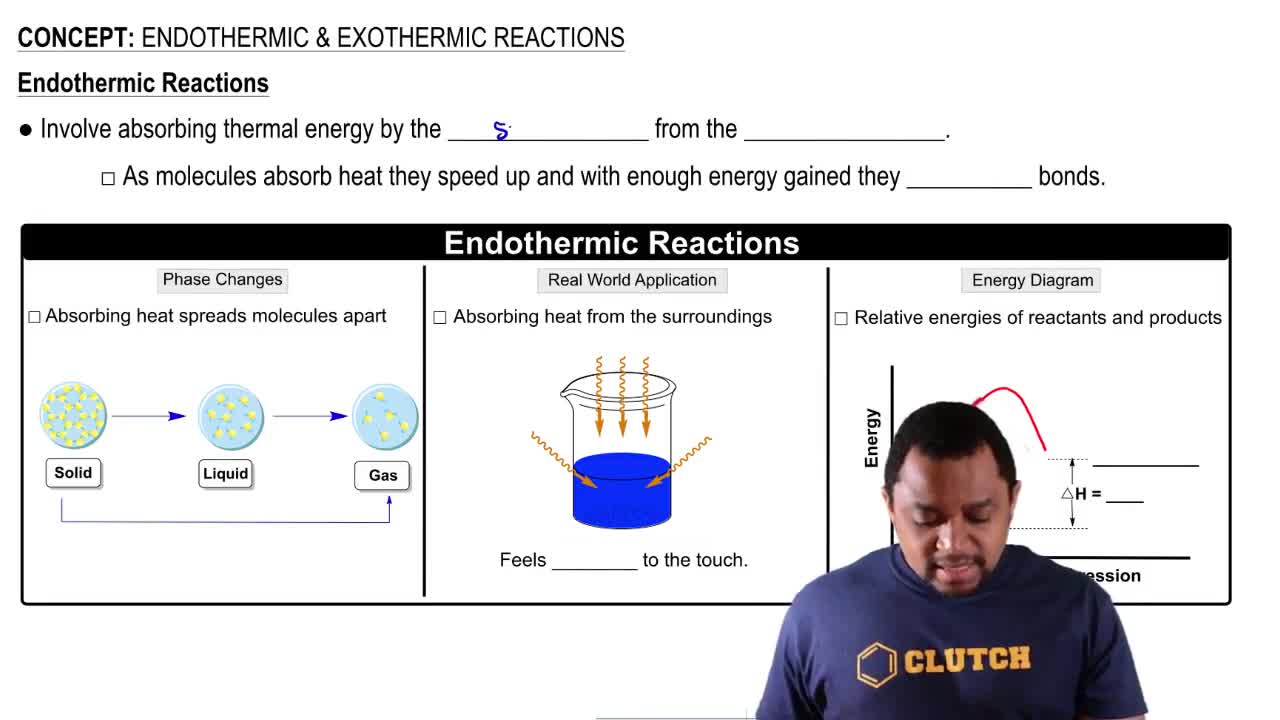
Two curves are shown in the following energy diagram:
b. Which curve represents the spontaneous reaction, and which the nonspontaneous?
The following diagram portrays a reaction of the type A(s) → B(g) + C(g), where the different-colored spheres represent different molecular structures. Assume that the reaction has ∆H = +9.1 kcal/mol (+38.1 kJ/mol).
a. What is the sign of ∆S for the reaction?
The following diagram portrays a reaction of the type A(s) → B(g) + C(g), where the different-colored spheres represent different molecular structures. Assume that the reaction has ∆H = +9.1 kcal/mol (+38.1 kJ/mol).
b. Is the reaction likely to be spontaneous at all temperatures, nonspontaneous at all temperatures, or spontaneous at some but nonspontaneous at others?
The vaporization of Br2 from the liquid to the gas state requires 7.4 kcal/mol (31.0 kJ/mol).
a. What is the sign of ∆H for this process? Write a reaction showing heat as a product or reactant.
The vaporization of Br2 from the liquid to the gas state requires 7.4 kcal/mol (31.0 kJ/mol).
c. How many kilojoules are needed to evaporate 82 g of Br2?