Is the total enthalpy (H) of the reactants for an endothermic reaction greater than or less than the total enthalpy of the products?

Converting liquid water to solid ice releases 1.44 kcal/mol (6.02 kJ/mol).How many kilocalories are released by freezing 32 g of H2O?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Molar Mass of Water

Phase Change and Latent Heat
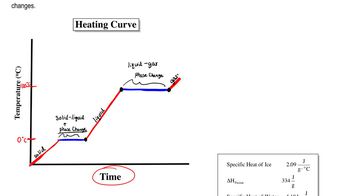
Energy Calculation

The vaporization of Br2 from the liquid to the gas state requires 7.4 kcal/mol (31.0 kJ/mol).
a. What is the sign of ∆H for this process? Write a reaction showing heat as a product or reactant.
The vaporization of Br2 from the liquid to the gas state requires 7.4 kcal/mol (31.0 kJ/mol).
c. How many kilojoules are needed to evaporate 82 g of Br2?
Acetylene (H–C≡C–H) is the fuel used in welding torches.
b. Estimate ∆H for this reaction (in kJ/mol) using the bond energies listed in Table 7.1.
Nitrogen in air reacts at high temperatures to form NO2 according to the following reaction: N2 + 2 O2 → 2 NO2
b. Estimate ∆H for this reaction (in kcal and kJ) using the bond energies from Table 7.1.
Glucose, also known as "blood sugar" when measured in blood, has the formula C6H12O6.
a. Write the equation for the combustion of glucose with O2 to give CO2 and H2O.
