Textbook Question
How many O atoms of mass 15.99 amu are in 15.99 g of oxygen?
1542
views

 Verified step by step guidance
Verified step by step guidance
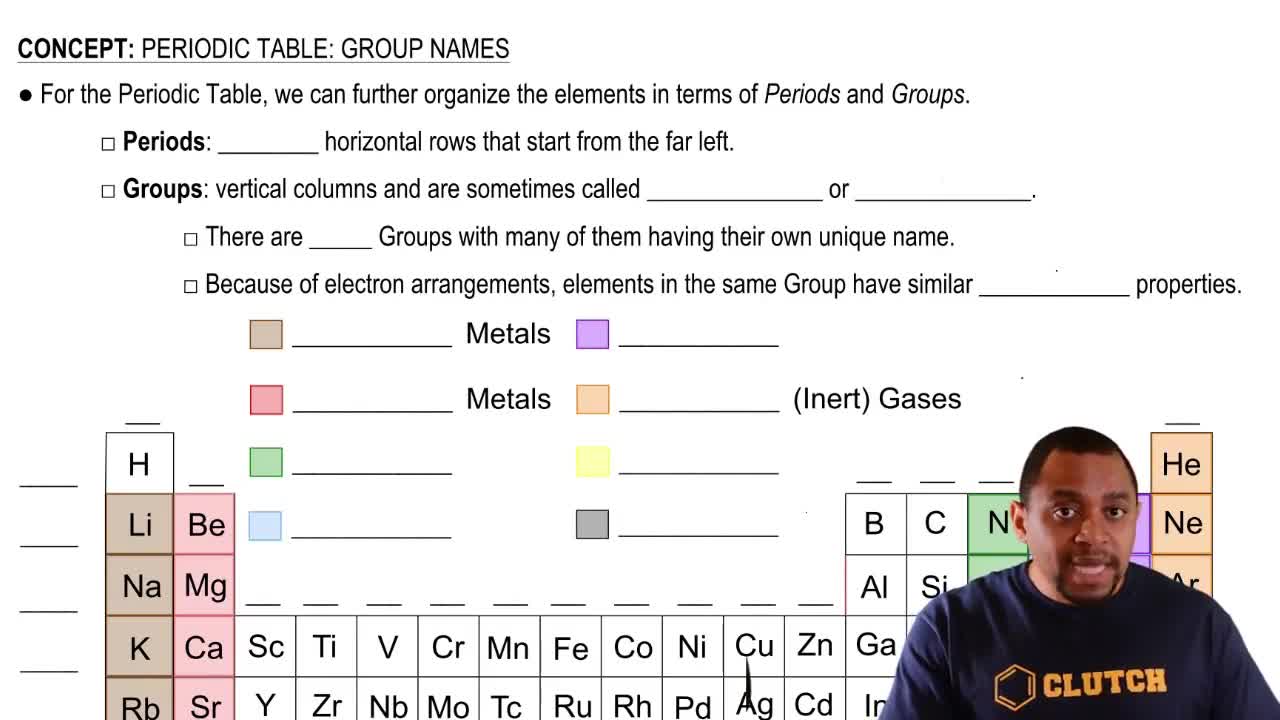
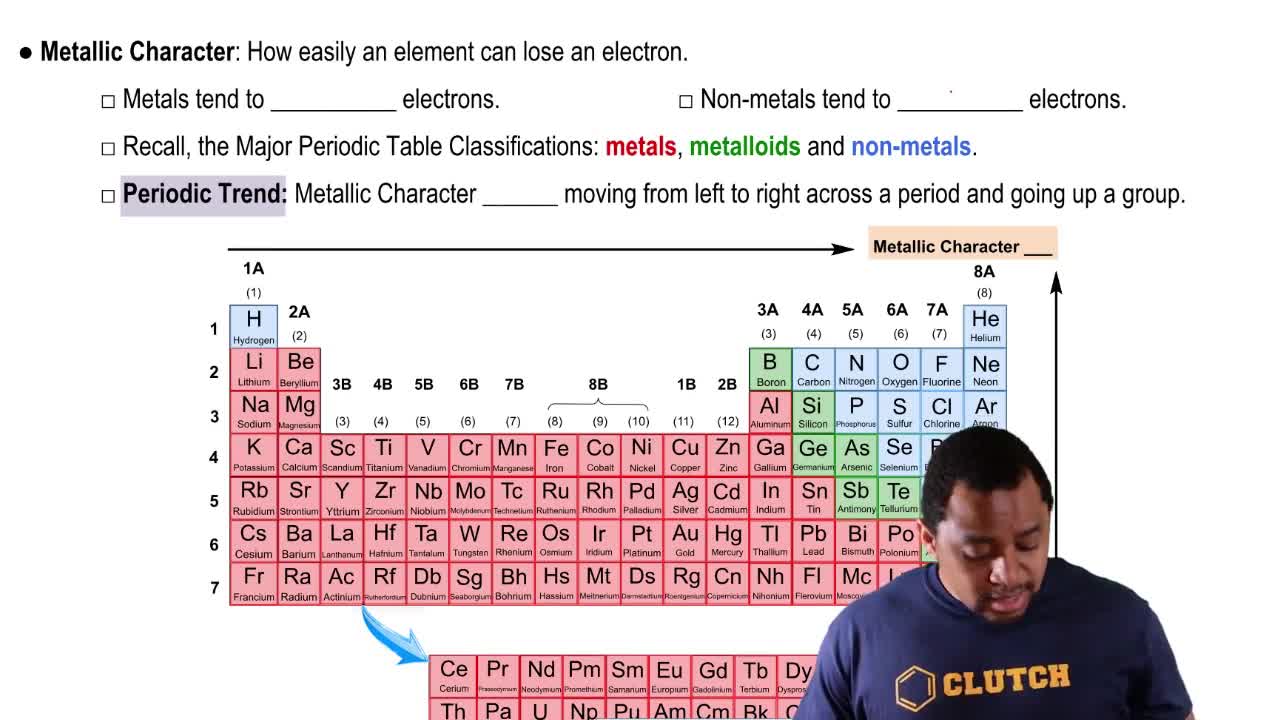

How many O atoms of mass 15.99 amu are in 15.99 g of oxygen?
Where within an atom are the three types of subatomic particles located?
Why does the fourth period in the periodic table contain 18 elements?
What is the total number of orbitals in the third shell? The fourth shell?
How many subshells are there in the third shell? The fourth shell? The fifth shell?
Use arrows to show electron pairing in the valence p subshell of
a. Sulfur
b. Bromine
c. Silicon