Textbook Question
Write condensed structures for the following molecular formulas. More than one isomer will be required for each.
a. Isomers of C8H18 that contain two methyl groups and a longest chain of 4 carbons
733
views

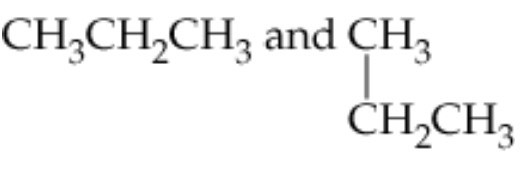
 Verified step by step guidance
Verified step by step guidance



Write condensed structures for the following molecular formulas. More than one isomer will be required for each.
a. Isomers of C8H18 that contain two methyl groups and a longest chain of 4 carbons
How many straight-chain isomers can you write that fit the following descriptions? See Worked Example 12.12 for guidance.
b. Amines (―NH2) with a longest chain of 7 carbons
How many isomers can you write that fit the following descriptions? See Worked Example 12.12 for guidance.
c. Alcohols (―OH) formed from 2-methylhexane
What is wrong with the following structures?
a. CH3=CHCH2CH2OH
There are two things wrong with the following structure. What are they?
What are the IUPAC names of the following alkanes?
f.