Give names or structures for the following ammonium salts. Indicate whether each is the ammonium salt of a primary, secondary, or tertiary amine.
c. N-Butyl-N-isopropylhexylammonium chloride

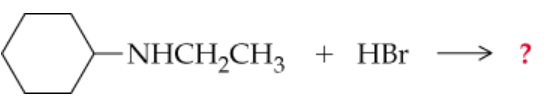
 Verified step by step guidance
Verified step by step guidance

Give names or structures for the following ammonium salts. Indicate whether each is the ammonium salt of a primary, secondary, or tertiary amine.
c. N-Butyl-N-isopropylhexylammonium chloride
Identify the functional groups in cocaine
Draw the structures of the ammonium ions formed when the amines in Problem 16.30 are treated with acid.
a. N-Methylpentylamine
b. N-Ethylcyclobutylamine
c. p-Propylaniline
Complete the following equations. (Hint: Remember that a nitrogen with three groups bound to it has a lone pair and one with four does not)
a.
Many hair conditioners contain an ammonium salt such as the following to help prevent 'fly-away' hair. These ions will react with neither acid nor base. Provide a reason why.
Choline has the following structure. Do you think that this substance reacts with aqueous hydrochloric acid? If so, what is the product? If not, why not?