Textbook Question
Draw structures for molecules that fit the following descriptions:
(c) C3H6O2 containing a carboxylic acid functional group
945
views

 Verified step by step guidance
Verified step by step guidance



Draw structures for molecules that fit the following descriptions:
(c) C3H6O2 containing a carboxylic acid functional group
Draw the straight-chain isomer with the formula (b) C9H20.
There are two branched-chain isomers with the formula C7H16, where the longest chain in the molecule is six carbons long. Draw them.
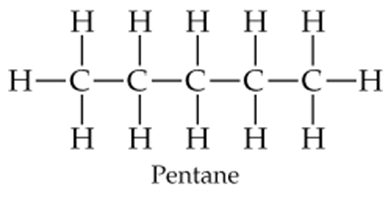
Draw the following three isomers of C5H12 as condensed structures:
b.
Draw the following three isomers of C5H12 as condensed structures:
c.
Convert the following line structures to condensed structures:
a.