How many different tripeptides that contain one leucine, one glutamate, and one tryptophan are possible?
Ch.10 Proteins Workers of the Cell

Frost4th EditionGeneral, Organic and Biological ChemistryISBN: 9780134988696Not the one you use?Change textbook
Chapter 6, Problem 25
Describe the differences in the shape of an α helix and a β-pleated sheet.
 Verified step by step guidance
Verified step by step guidance1
Understand that both α helix and β-pleated sheet are types of secondary structures found in proteins, stabilized by hydrogen bonds.
Recognize that an α helix is a right-handed coil or spiral structure, where the backbone of the polypeptide chain forms the inner part of the helix and the side chains extend outward.
Note that in an α helix, hydrogen bonds form between the carbonyl oxygen of one amino acid and the amide hydrogen of an amino acid four residues earlier, creating a tightly packed structure.
Identify that a β-pleated sheet consists of two or more polypeptide chains (or segments of a single chain) aligned next to each other, forming a sheet-like structure.
Observe that in a β-pleated sheet, hydrogen bonds form between the carbonyl oxygen of one chain and the amide hydrogen of an adjacent chain, resulting in a zigzag or pleated appearance.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
α Helix Structure
The α helix is a common secondary structure in proteins characterized by a right-handed coil or spiral. Each turn of the helix typically consists of about 3.6 amino acids, with hydrogen bonds forming between the carbonyl oxygen of one amino acid and the amide hydrogen of another, four residues down the chain. This structure provides stability and is often found in fibrous proteins.
Recommended video:
Guided course
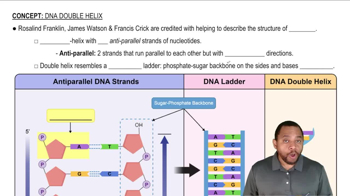
DNA Double Helix Concept 1
β-Pleated Sheet Structure
The β-pleated sheet is another type of secondary structure in proteins, formed by linking two or more strands of amino acids through hydrogen bonds. These strands can be parallel or antiparallel, creating a sheet-like appearance. The pleated nature allows for a more extended conformation, contributing to the overall stability and functionality of the protein.
Recommended video:
Guided course
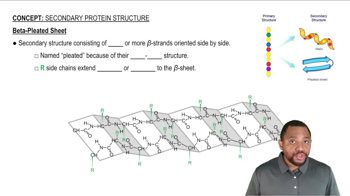
Beta-Pleated Sheet Concept 4
Hydrogen Bonding in Protein Structures
Hydrogen bonding is a crucial interaction that stabilizes both α helices and β-pleated sheets in protein structures. In α helices, hydrogen bonds form within the same strand, while in β-pleated sheets, they occur between different strands. This bonding is essential for maintaining the three-dimensional shape of proteins, influencing their biological activity and interactions.
Recommended video:
Guided course

Summary of Protein Structure Example 1
Related Practice
Textbook Question
633
views
Textbook Question
Name the stabilizing attractive force found in secondary structures of proteins.
655
views
Textbook Question
When a protein folds into its tertiary structure, does the primary structure change? Explain.
805
views
Textbook Question
What type of interaction would you expect between the side chains of each of the following pairs of amino acids in the tertiary structure of a protein?
b. alanine and valine
534
views
Textbook Question
What type of interaction would you expect between the side chains of each of the following pairs of amino acids in the tertiary structure of a protein?
a. lysine and glutamate
819
views
Textbook Question
What type of interaction would you expect between the side chains of each of the following pairs of amino acids in the tertiary structure of a protein?
b. leucine and isoleucine
570
views
