Textbook Question
What is the one-letter amino acid sequence formed from the following mRNA that codes for a pentapeptide that is an endorphin called Met-enkephalin?
5'AUG|UAC|GGU|GGA|UUU|AUG|UAA3'
771
views

 Verified step by step guidance
Verified step by step guidance


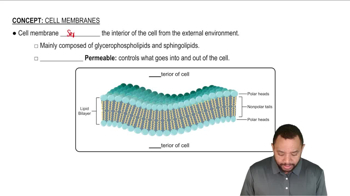
What is the one-letter amino acid sequence formed from the following mRNA that codes for a pentapeptide that is an endorphin called Met-enkephalin?
5'AUG|UAC|GGU|GGA|UUU|AUG|UAA3'
What is the anticodon on tRNA for each of the following codons in an mRNA?
c. GAA
A base substitution changes a codon for an enzyme from GCC to GCA. Why is there no change in the amino acid order in the protein?
A base substitution for an enzyme replaces leucine (a nonpolar amino acid) with alanine. Why does this change in amino acids have little effect on the biological activity of the enzyme?
Provide a reason why there is no vaccine for the common cold.
How is CRISPR different from earlier recombinant DNA techniques?