Textbook Question
Write the products of the following reactions:
(b)
701
views

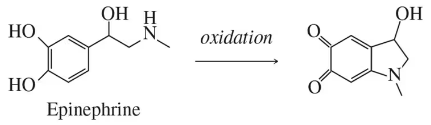
 Verified step by step guidance
Verified step by step guidance



Write the products of the following reactions:
(b)
Write the products of the following reactions:
(a)
Fill in the missing organic products or reactants for the following hydrogenation reactions:
(a)
Fill in the missing organic products for the complete hydrogenation of the following:
(a)
Fill in the missing organic products for the complete hydrogenation of the following:
(c)
Fill in the missing organic product or reactant for the following hydration reactions:
(b)