Textbook Question
Considering their chemical structure, why are the melting points of oils lower than those of fats?
520
views

 Verified step by step guidance
Verified step by step guidance


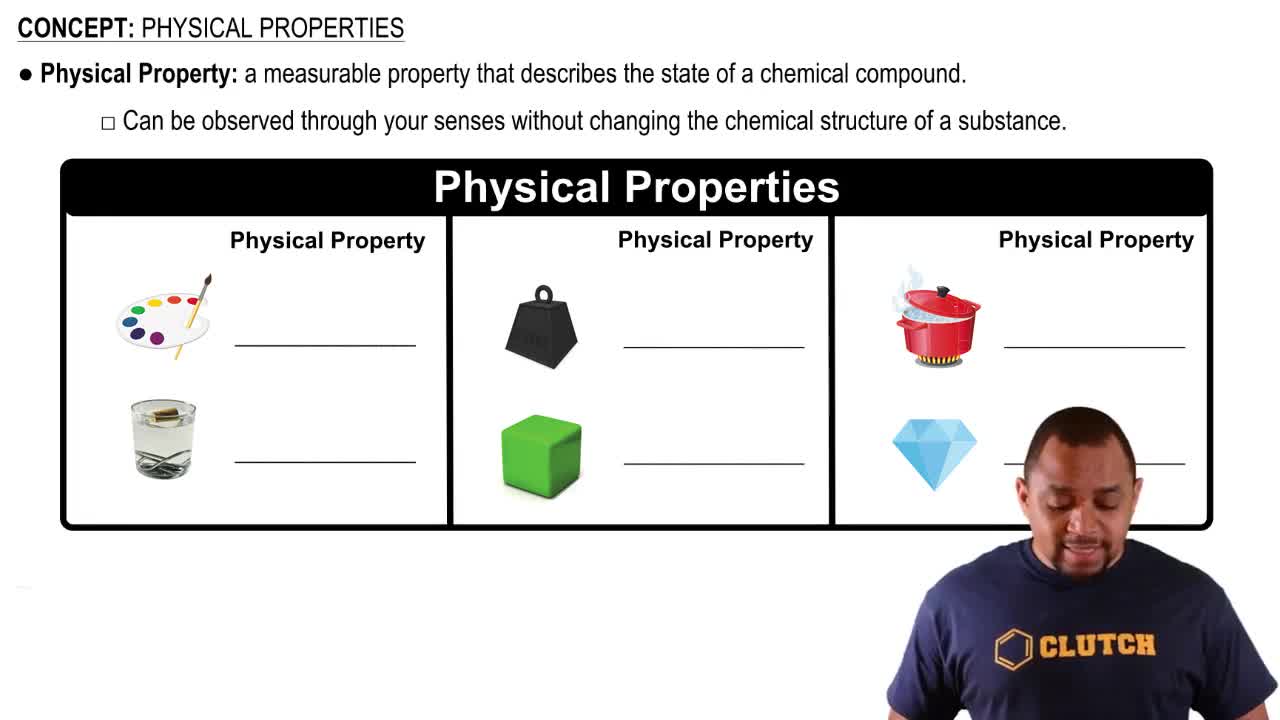
Considering their chemical structure, why are the melting points of oils lower than those of fats?
One of the main triglycerides in palm oil is tripalmitin. It contains three fatty acids with carbon designations of, [16:0]. Draw the structure of tripalmitin. Would you expect this molecule to be a solid or liquid at room temperature?
Draw a possible hydrogen bond between a molecule of cholesterol and a molecule of water.
Compare the structure of a soap molecule to a phospholipid and explain why a soap’s polar head is smaller than that of a phospholipid.