Calculate the specific heat of copper if it takes 23 cal (96 J) to heat a 5.0 g sample from 25 °C to 75 °C.

A white solid with a melting point of 730 °C is melted. When electricity is passed through the resultant liquid, a brown gas and a molten metal are produced. Neither the metal nor the gas can be broken down into anything simpler by chemical means. Classify each—the white solid, the molten metal, and the brown gas—as a mixture, a compound, or an element.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Elements, Compounds, and Mixtures

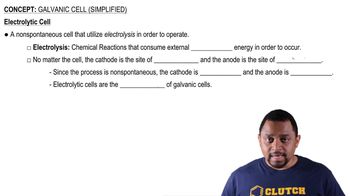
Electrolysis
Physical and Chemical Properties

The specific heat of fat is 0.45 cal/(g ⋅ °C) (1.9 J/g °C) and the density of fat is 0.94 g/cm3. How much energy (in calories and joules) is needed to heat 10 cm3 of fat from room temperature (25 °C) to its melting point (35 °C)?
When 100 cal (418 J) of heat is applied to a 125 g sample, the temperature increases by 28 °C. Calculate the specific heat of the sample and compare your answer to the values in Table 1.10. What is the identity of the sample?
Refer to the pencil in Problem 1.31. Using the equivalent values in Table 1.8 as conversion factors, convert the length measured in inches to centimeters. Compare the calculated length in centimeters to the length in centimeters measured using the metric ruler. How do the two values compare? Explain any differences.
<IMAGE>
Gemstones are weighed in carats, where 1 carat = 200 mg exactly. What is the mass in grams of the Hope diamond, the world's largest blue diamond, at 44.4 carats?
The relationship between the nutritional unit for energy and the metric unit is 1 Calorie = 1 kcal.
a. One donut contains 350 Calories. Convert this to calories and joules.
