Heating and cooling curves are essential tools for understanding the heat absorbed or released during phase changes of substances, such as water. A heating curve illustrates how temperature changes over time as heat is added. For water, key temperatures to remember are 0 degrees Celsius, where it freezes or melts, and 100 degrees Celsius, where it begins to boil or condense. These temperatures are critical for identifying phase changes, which are represented by flat sections on the curve where temperature remains constant.
Below 0 degrees Celsius, water exists as a solid (ice). At 0 degrees Celsius, the solid begins to melt, transitioning into a liquid. This melting phase is characterized by a plateau on the curve, indicating that while heat is being added, the temperature does not increase until all the ice has melted. Once fully melted, the temperature of the liquid water begins to rise until it reaches 100 degrees Celsius, where it starts to boil. During boiling, the curve again flattens, indicating a phase change from liquid to gas (vapor). After all the liquid has evaporated, the temperature of the gas continues to increase.
During phase changes, the average kinetic energy of the particles remains constant, as indicated by the flat sections of the curve. This is because the heat energy being added is used to overcome intermolecular forces rather than increasing temperature. In contrast, during temperature changes, heat energy is converted into kinetic energy, resulting in an increase in temperature and average kinetic energy of the substance.
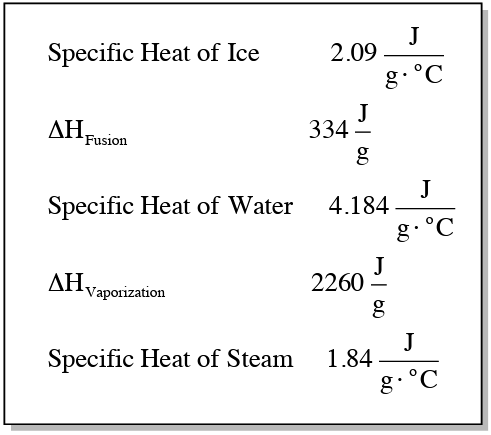
To quantify the heat transfer during these processes, different equations are used. For segments where temperature changes occur, the equation is given by:
\[ q = mc\Delta T \]
where \( q \) is the heat absorbed or released, \( m \) is the mass, \( c \) is the specific heat capacity, and \( \Delta T \) is the change in temperature (final temperature minus initial temperature). For phase changes, where temperature remains constant, the equation simplifies to:
\[ q = m \Delta H \]
Here, \( \Delta H \) represents the enthalpy change associated with the phase transition, such as fusion (melting) or vaporization (boiling). The specific heat values differ depending on the phase of the substance: solid, liquid, or gas.
Understanding these concepts is crucial for analyzing heating and cooling curves, as they provide insight into the energy changes that occur during phase transitions and temperature changes. The cooling curve, which represents the reverse process, can be analyzed similarly, with the same principles applying in the opposite direction.