The reaction CO(g) + H2O(g) ⇌ CO2(g) + H2(g) has ∆H = -9.8 kcal/mol (-41 kJ/mol). Does the amount of H2 in an equilibrium mixture increase or decrease when the temperature is decreased?

The reaction Fe3+(aq) + Cl-(aq) ⇌ FeCl2+(aq) is endothermic. How will the equilibrium concentration of FeCl2+ change when
b. Cl- is precipitated by addition of AgNO3?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Le Chatelier's Principle
Equilibrium Constant (K)

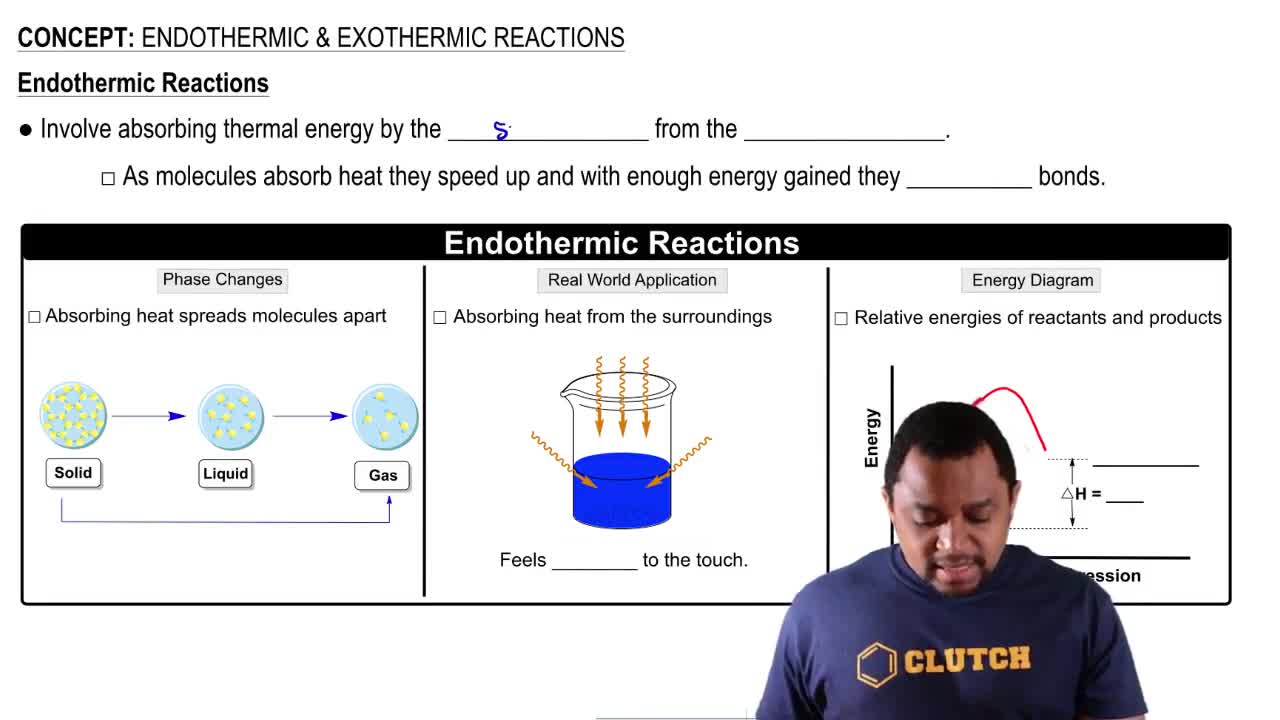
Endothermic Reactions
The reaction H2(g) + I2(g) ⇌ 2 HI(g) has ∆H = -2.2 kcal/mol (-9.2 kJ/mol). Will the equilibrium concentration of HI increase or decrease when
b. H2 is removed?
The reaction H2(g) + I2(g) ⇌ 2 HI(g) has ∆H = -2.2 kcal/mol (-9.2 kJ/mol). Will the equilibrium concentration of HI increase or decrease when
c. A catalyst is added?
For the unbalanced combustion reaction shown, 1 mol of ethanol, C2H5OH, releases 327 kcal (1370 kJ):
C2H5OH + O2 → CO2 + H2O
e. If the density of ethanol is 0.789 g/mL, calculate the combustion energy of ethanol in kilocalories/milliliter and kilojoules/milliliter
For the production of ammonia from its elements, ∆H = -22 kcal/mol(-19 kJ/mol).
b. How much energy (in kilocalories and kilojoules) is involved in the production of 0.700 mol of NH3?
Magnetite, an iron ore with formula Fe3O4, can be reduced by treatment with hydrogen to yield iron metal and water vapor.
a. Write the balanced equation.
