If you had only 23 g of KOH remaining in a bottle, how many milliliters of 10.0% (m/v) solution could you prepare? How many milliliters of 0.25 M solution?
Ch.9 Solutions

Chapter 9, Problem 63
An aqueous solution that contains 285 ppm of potassium nitrate (KNO3) is being used to feed plants in a garden. What volume of this solution is needed to prepare 2.0 L of a solution that is 75 ppm in KNO3?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the concept of ppm (parts per million). It is a way to express concentration, where 1 ppm means 1 part of solute per 1,000,000 parts of solution. In this problem, the concentration of potassium nitrate (KNO₃) is given in ppm for two solutions: 285 ppm (initial solution) and 75 ppm (final solution).
Step 2: Use the dilution equation to relate the concentrations and volumes of the solutions. The dilution equation is: , where C1 and C2 are the initial and final concentrations, and V1 and V2 are the initial and final volumes, respectively.
Step 3: Substitute the known values into the dilution equation. Here, C1 = 285 ppm, C2 = 75 ppm, and V2 = 2.0 L. The equation becomes: .
Step 4: Solve for V1, the volume of the initial solution needed. Rearrange the equation to isolate V1: .
Step 5: Perform the calculation to determine the value of V1. This will give you the volume of the 285 ppm solution required to prepare 2.0 L of a 75 ppm solution.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Parts Per Million (ppm)
Parts per million (ppm) is a unit of measurement that expresses the concentration of a substance in a solution. It indicates how many parts of a solute are present in one million parts of the solution. For example, a concentration of 285 ppm means that there are 285 grams of solute in one million grams of solution, which is crucial for understanding the dilution process in this question.
Recommended video:
Guided course

Parts per Million (ppm) Concept 1
Dilution
Dilution is the process of reducing the concentration of a solute in a solution, typically by adding more solvent. In this context, to prepare a 75 ppm solution from a 285 ppm solution, one must calculate the appropriate volume of the concentrated solution to mix with water. The dilution formula, C1V1 = C2V2, where C is concentration and V is volume, is essential for solving this problem.
Recommended video:
Guided course
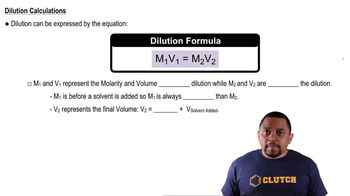
Dilutions
Concentration Calculation
Concentration calculation involves determining the amount of solute in a given volume of solution. In this scenario, it is necessary to calculate how much of the 285 ppm solution is required to achieve a final concentration of 75 ppm in a total volume of 2.0 L. This requires understanding the relationship between the initial and final concentrations and the volumes involved.
Recommended video:
Guided course

Percent Concentrations Concept 1
Related Practice
Textbook Question
2038
views
Textbook Question
Nalorphine, a relative of morphine, is used to combat withdrawal symptoms in heroin users. How many milliliters of a 0.40% (m/v) solution of nalorphine must be injected to obtain a dose of 1.5 mg?
1107
views
Textbook Question
Sodium thiosulfate (Na2S2O3) the major component in photographic fixer solution, reacts with silver bromide to dissolve it according to the following reaction:
AgBr(s) + 2 Na2S2O3(aq) → Na3Ag(S2O3)2(aq) + NaBr(aq)
b. How many mL of 0.02 M Na2S2O3 contain this number of moles?
1717
views
Textbook Question
What is the concentration of a NaCl solution, in (m/v)%, prepared by diluting 65 mL of a saturated solution, which has a concentration of 37 (m/v)%, to 480 mL?
2396
views
Textbook Question
What is an electrolyte?
2572
views
Textbook Question
What does it mean when we say that the concentration of Ca2+ in blood is 3.0 mEq/L?
1599
views
