
The diagram to the right shows plots of vapor pressure versus temperature for a solvent and a solution.
b. What is the approximate boiling-point elevation for the solution?
<IMAGE>
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Vapor Pressure

Boiling Point Elevation

Colligative Properties
The typical concentration of Mg2+ in blood is 3 mEq/L. How many milligrams of Mg2+ are in 250 mL of blood?
When 1.0 mol of HF is dissolved in 1.0 kg of water, the boiling point of the resulting solution is 100.5 °C. Is HF a strong or weak electrolyte? Explain.
The diagram to the right shows plots of vapor pressure versus temperature for a solvent and a solution.
c. What is the approximate concentration of the solution in mol/kg, if 1 mol of solute particles raises the boiling point of 1 kg of solvent by 3.63 °C?
<IMAGE>
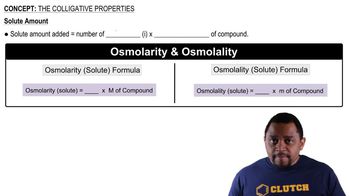
What is the osmolarity of the following solutions?
a. 0.35 M KBr
b. 0.15 M glucose + 0.05 M K2SO4
A typical oral rehydration solution (ORS) for infants contains 90 mEq/L Na+ , 20 mEq/L K+ , 110 mEq/L Cl- , and 2.0% (m/v) glucose (MW = 180g/mol)
a. Calculate the concentration of each ORS component in units of molarity.
