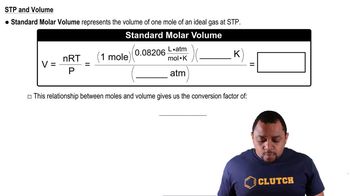
At a total atmospheric pressure of 1.00 atm, the partial pressure of CO2 in air is approximately 4.0 × 10-4atm. Using the data in Problem 9.4, what is the solubility of CO2 in an open bottle of seltzer water at 20 °C?

The Environmental Protection Agency has set the limit for arsenic in drinking water at 0.010 ppm. To what volume would you need to dilute 1.5 L of water containing 5.0 ppm arsenic to reach the acceptable limit?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
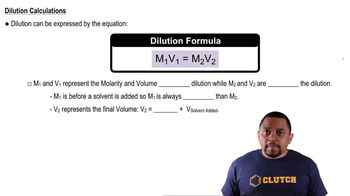
Dilution Principle
Parts Per Million (ppm)

Environmental Standards
The maximum amounts of lead and copper allowed in drinking water are 0.015 mg/kg for lead and 1.3 mg/kg for copper. Express these values in parts per million, and tell the maximum amount of each (in grams) allowed in 100 g of water.
The concentration of cholesterol (C27H46O) in blood is approximately 5.0 mM. How many grams of cholesterol are in 250 mL of blood?
The typical concentration of Mg2+ in blood is 3 mEq/L. How many milligrams of Mg2+ are in 250 mL of blood?
When 1.0 mol of HF is dissolved in 1.0 kg of water, the boiling point of the resulting solution is 100.5 °C. Is HF a strong or weak electrolyte? Explain.
The diagram to the right shows plots of vapor pressure versus temperature for a solvent and a solution.
b. What is the approximate boiling-point elevation for the solution?
<IMAGE>
