The concentration of cholesterol (C27H46O) in blood is approximately 5.0 mM. How many grams of cholesterol are in 250 mL of blood?
Ch.9 Solutions

Chapter 9, Problem 21
When 1.0 mol of HF is dissolved in 1.0 kg of water, the boiling point of the resulting solution is 100.5 °C. Is HF a strong or weak electrolyte? Explain.
 Verified step by step guidance
Verified step by step guidance1
Determine the boiling point elevation (ΔT_b) by subtracting the boiling point of pure water (100.0 °C) from the boiling point of the solution (100.5 °C). This gives ΔT_b = 0.5 °C.
Use the boiling point elevation formula: , where is the van 't Hoff factor, is the boiling point elevation constant for water (0.512 °C·kg/mol), and is the molality of the solution.
Calculate the molality () of the solution using the formula . Here, the moles of HF are 1.0 mol, and the mass of water is 1.0 kg, so .
Rearrange the boiling point elevation formula to solve for the van 't Hoff factor (): . Substitute the known values: , , and .
Interpret the value of . If is close to 1, HF is a weak electrolyte because it does not fully dissociate in water. If is significantly greater than 1, HF is a strong electrolyte because it dissociates into ions completely.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electrolytes
Electrolytes are substances that dissociate into ions when dissolved in water, allowing the solution to conduct electricity. They can be classified as strong or weak based on their degree of ionization. Strong electrolytes completely dissociate into ions, while weak electrolytes only partially dissociate, resulting in fewer ions in solution.
Recommended video:
Guided course
Electrolytes (Simplified) Example 3
Boiling Point Elevation
Boiling point elevation is a colligative property that describes how the boiling point of a solvent increases when a solute is added. The extent of this elevation depends on the number of solute particles in the solution. In this case, the increase in boiling point from 100 °C to 100.5 °C indicates that the solute (HF) contributes to the solution's overall particle concentration.
Recommended video:
Guided course

Boiling Point Elevation Concept 1
HF as an Electrolyte
Hydrofluoric acid (HF) is classified as a weak electrolyte because it only partially ionizes in solution. When HF dissolves in water, it produces a mixture of HF molecules and ions (H⁺ and F⁻), but not all HF molecules dissociate. The observed boiling point elevation suggests that HF does contribute to the solution's ionic strength, but its incomplete dissociation confirms its status as a weak electrolyte.
Recommended video:
Guided course
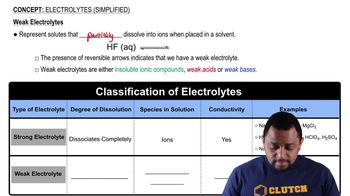
Electrolytes (Simplified) Concept 2
Related Practice
Textbook Question
2081
views
Textbook Question
The Environmental Protection Agency has set the limit for arsenic in drinking water at 0.010 ppm. To what volume would you need to dilute 1.5 L of water containing 5.0 ppm arsenic to reach the acceptable limit?
1776
views
Textbook Question
The typical concentration of Mg2+ in blood is 3 mEq/L. How many milligrams of Mg2+ are in 250 mL of blood?
1733
views
Textbook Question
The diagram to the right shows plots of vapor pressure versus temperature for a solvent and a solution.
b. What is the approximate boiling-point elevation for the solution?
<IMAGE>
2084
views
Textbook Question
The diagram to the right shows plots of vapor pressure versus temperature for a solvent and a solution.
c. What is the approximate concentration of the solution in mol/kg, if 1 mol of solute particles raises the boiling point of 1 kg of solvent by 3.63 °C?
<IMAGE>
2179
views
Textbook Question
What is the osmolarity of the following solutions?
a. 0.35 M KBr
b. 0.15 M glucose + 0.05 M K2SO4
1488
views
