Textbook Question
The following alkenes can be prepared by dehydration of an appropriate alcohol. Show the structure of the alcohol in each case that would provide the alkene shown as the major product.
e. 1,4-Pentadiene
647
views

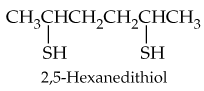
 Verified step by step guidance
Verified step by step guidance



The following alkenes can be prepared by dehydration of an appropriate alcohol. Show the structure of the alcohol in each case that would provide the alkene shown as the major product.
e. 1,4-Pentadiene
What alcohols would you oxidize to obtain the following carbonyl compounds?
a.
b.
c.
What is the structural relationship between a thiol and an alcohol?
The boiling point of propanol is 97 °C ,much higher than that of either ethanethiol (37 °C) or chloroethane (13 °C) , even though all three compounds have similar MWs. Explain.
Propanol is very soluble in water, but ethanethiol and chloroethane are only slightly soluble. Explain.
Define the following terms:
b. Achiral