Textbook Question
Identify the chiral center(s) in each of the following molecules:
a. 2-Methyl-3-pentanol
940
views

 Verified step by step guidance
Verified step by step guidance

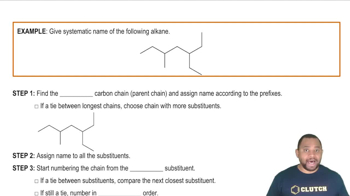

Identify the chiral center(s) in each of the following molecules:
a. 2-Methyl-3-pentanol
Are the following molecules chiral or achiral? If they are chiral, identify the chiral carbon atom(s).
d.
Name all unbranched ether and alcohol isomers with formula C5H12O and write their structural formulas.
Which of the alcohols pictured in Problem 14.48 are chiral? Indicate the chiral carbons for those that are chiral.
a.
b.
c. 2,3-Pentanediol
d.
e.
f.
Write the formulas and IUPAC names for the following common alcohols.
a. Rubbing alcohol
Write the formulas and IUPAC names for the following common alcohols.
d. Diol used as antifreeze (two answers)