Textbook Question
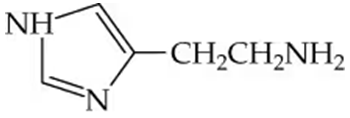
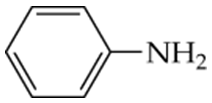
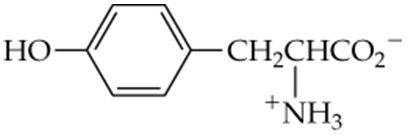
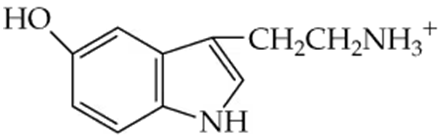
Arrange the following compounds in order of increasing boiling point. Explain why you placed them in that order.
a.
747
views

 Verified step by step guidance
Verified step by step guidance

Arrange the following compounds in order of increasing boiling point. Explain why you placed them in that order.
a.
Draw the structures of (a) ethylamine and (b) trimethylamine. Use dashed lines to show how they would form hydrogen bonds to water molecules.
Provide compounds that fit the following descriptions:
a. Two amines that are gases at room temperature
b. A heterocyclic amine
c. A compound with an amine group on an aromatic ring
Write an equation for the acid-base equilibrium of:
a.Pyrrolidine and water
Label each species in the equilibrium as either an acid or a base.
Complete the following equations:
a.
Complete the following equations:
c.