Textbook Question
Give systematic names for the following structures and structures for the names:
b. N-Phenylacetamide
755
views

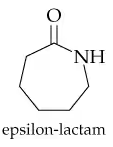
 Verified step by step guidance
Verified step by step guidance

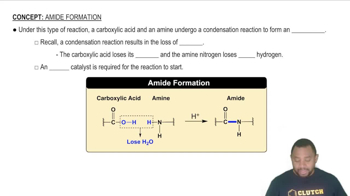
Give systematic names for the following structures and structures for the names:
b. N-Phenylacetamide
Household soap is a mixture of the sodium or potassium salts of long-chain carboxylic acids that arise from saponification of animal fat.
b. Draw the structures of the soap molecules produced in the following reaction:
A simple polyamide can be made from ethylenediamine and oxalic acid (Table 17.1). Draw the polymer formed when three units of ethylenediamine reacts with two units of oxalic acid.
In the following compound
a. Identify the phosphate ester linkage.