Give an example of a protein containing primarily alpha-helices. Is this a fibrous or globular protein?
Ch.18 Amino Acids and Proteins

Chapter 18, Problem 77
Is the bond formed between each pair in Problem 18.76 covalent or noncovalent?
a. Cysteine and cysteine
b. Alanine and leucine
c. Aspartic acid and asparagine
d. Serine and lysine
 Verified step by step guidance
Verified step by step guidance1
Identify the elements in each pair from Problem 18.76 and determine their positions on the periodic table. This will help assess their electronegativity values.
Recall that a covalent bond is formed when two atoms share electrons, typically occurring between nonmetals with similar electronegativity values. Noncovalent interactions, on the other hand, include ionic bonds (formed between metals and nonmetals) and other weaker interactions like hydrogen bonds or van der Waals forces.
Compare the electronegativity values of the two elements in each pair. If the difference in electronegativity is small (generally less than 1.7), the bond is likely covalent. If the difference is large, the bond is likely ionic (a type of noncovalent interaction).
Consider the type of elements involved: if both are nonmetals, the bond is likely covalent. If one is a metal and the other is a nonmetal, the bond is likely ionic.
Classify each pair based on the above criteria, labeling the bond as covalent or noncovalent (ionic or other noncovalent interaction).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Covalent Bonds
Covalent bonds are formed when two atoms share one or more pairs of electrons, resulting in a stable balance of attractive and repulsive forces between the atoms. This type of bond typically occurs between nonmetals and is characterized by the strength and stability it provides to molecules. An example is the bond between hydrogen and oxygen in water (H2O).
Recommended video:
Guided course

Covalent Bonds Example 1
Noncovalent Interactions
Noncovalent interactions are weaker than covalent bonds and do not involve the sharing of electrons. These interactions include hydrogen bonds, ionic bonds, van der Waals forces, and hydrophobic interactions. They play crucial roles in biological processes, such as the folding of proteins and the binding of substrates to enzymes.
Recommended video:
Guided course
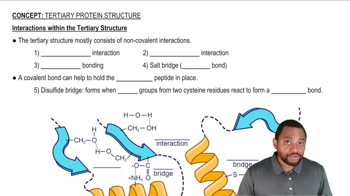
Interactions within the Tertiary Structure Concept 2
Bond Polarity
Bond polarity refers to the distribution of electrical charge over the atoms joined by the bond. In covalent bonds, if the atoms have different electronegativities, the shared electrons may be pulled closer to one atom, creating a polar covalent bond. Understanding bond polarity is essential for determining the nature of interactions between molecules, including whether they are covalent or noncovalent.
Recommended video:
Guided course

Molecular Polarity (Simplified) Concept 1
Related Practice
Textbook Question
409
views
Textbook Question
What kind of bond would you expect between the side chains of the following amino acids?
b. Alanine and leucine
758
views
Textbook Question
What kind of bond would you expect between the side chains of the following amino acids?
c. Aspartic acid and asparagine
687
views
Textbook Question
What is meant by the following terms as they apply to protein structure, and what bonds or molecular interactions stabilize that level of structure?
a. Primary structure
763
views
Textbook Question
What is meant by the following terms as they apply to protein structure, and what bonds or molecular interactions stabilize that level of structure?
b. Secondary structure
619
views
Textbook Question
What is meant by the following terms as they apply to protein structure, and what bonds or molecular interactions stabilize that level of structure?
d. Quaternary structure
721
views
