Proteins are usually least soluble in water at their isoelectric points. Explain.
Ch.18 Amino Acids and Proteins

Chapter 18, Problem 61
Write structural formulas for the two dipeptides that contain leucine and aspartate.
 Verified step by step guidance
Verified step by step guidance1
Understand that a dipeptide is formed by linking two amino acids through a peptide bond, which is a covalent bond formed between the carboxyl group of one amino acid and the amino group of another amino acid, with the release of a water molecule.
Identify the structures of leucine and aspartate. Leucine is a nonpolar, hydrophobic amino acid with the side chain -CH2-CH-(CH3)2, while aspartate is a polar, negatively charged amino acid with the side chain -CH2-COO⁻.
Determine the two possible sequences of the dipeptide: (1) Leucine as the N-terminal amino acid and aspartate as the C-terminal amino acid, and (2) Aspartate as the N-terminal amino acid and leucine as the C-terminal amino acid. The N-terminal refers to the amino acid with the free amino group, and the C-terminal refers to the amino acid with the free carboxyl group.
Draw the structural formula for the first dipeptide (Leu-Asp). Start with the amino group of leucine, connect it to the carboxyl group of aspartate via a peptide bond, and ensure the side chains of leucine and aspartate are correctly represented.
Draw the structural formula for the second dipeptide (Asp-Leu). Start with the amino group of aspartate, connect it to the carboxyl group of leucine via a peptide bond, and ensure the side chains of aspartate and leucine are correctly represented.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Dipeptides
Dipeptides are organic compounds formed by the condensation of two amino acids. In this process, the carboxyl group of one amino acid reacts with the amino group of another, releasing a molecule of water and forming a peptide bond. Understanding the structure and formation of dipeptides is essential for drawing their structural formulas.
Recommended video:
Guided course
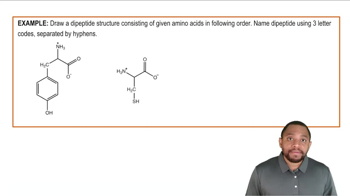
Peptides Example 2
Amino Acids
Amino acids are the building blocks of proteins, consisting of a central carbon atom, an amino group, a carboxyl group, a hydrogen atom, and a variable R group that determines the specific properties of each amino acid. Leucine and aspartate are two distinct amino acids, with leucine being non-polar and hydrophobic, while aspartate is polar and negatively charged. Recognizing their structures is crucial for constructing the correct dipeptide formulas.
Recommended video:
Guided course

Amino Acid Catabolism: Amino Group Example 2
Structural Formulas
Structural formulas represent the arrangement of atoms within a molecule, illustrating how atoms are bonded together. For dipeptides, the structural formula will show the sequence of amino acids and the peptide bond formed between them. Accurately depicting these structures is vital for understanding the chemical properties and functions of the resulting dipeptides.
Recommended video:
Guided course

Structural Formula Concept 2
Related Practice
Textbook Question
1319
views
Textbook Question
How could you make the zwitterion of aspartic acid more soluble in water?
832
views
Textbook Question
Use the three-letter abbreviations to name all tripeptides that contain valine, methionine, and leucine.
341
views
Textbook Question
The endorphins are a group of naturally occurring neurotransmitters that act in a manner similar to morphine to control pain. Research has shown that the biologically active parts of the endorphin molecules are simple pentapeptides called enkephalins. Draw the structure of the methionine enkephalin with the sequence Tyr-Gly-Gly-Phe-Met. Identify the N-terminal and C-terminal amino acids.
357
views
Textbook Question
Identify the amino acids present in the peptide shown and name the peptide using the three-letter abbreviations.
306
views
Textbook Question
Identify the N-terminal and C-terminal amino acids of the peptide.
629
views
