Which of the following forms of aspartic acid would you expect to predominate at low pH, neutral pH, and high pH?
a.
b.
c.

 Verified step by step guidance
Verified step by step guidance
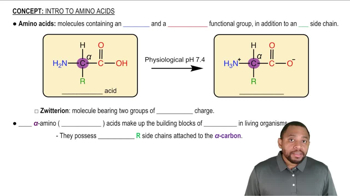


Which of the following forms of aspartic acid would you expect to predominate at low pH, neutral pH, and high pH?
a.
b.
c.
Which form of aspartic acid in Problem 18.54 is the zwitterion? What is the pI for the zwitterion?
a.
b.
c.
Proteins are usually least soluble in water at their isoelectric points. Explain.
Use the three-letter abbreviations to name all tripeptides that contain valine, methionine, and leucine.
Write structural formulas for the two dipeptides that contain leucine and aspartate.
The endorphins are a group of naturally occurring neurotransmitters that act in a manner similar to morphine to control pain. Research has shown that the biologically active parts of the endorphin molecules are simple pentapeptides called enkephalins. Draw the structure of the methionine enkephalin with the sequence Tyr-Gly-Gly-Phe-Met. Identify the N-terminal and C-terminal amino acids.