In solution, glucose exists predominantly in the cyclic hemiacetal form, which does not contain an aldehyde group. How is it possible for mild oxidizing agents to oxidize glucose?
Ch.20 Carbohydrates

Chapter 20, Problem 32
How many chiral carbon atoms are present in each of the molecules shown in Problem 20.31?
a. 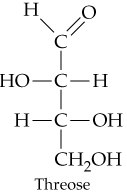
b. 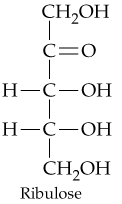
c. 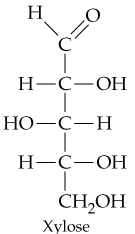
d. 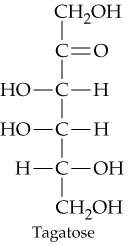
 Verified step by step guidance
Verified step by step guidance1
Identify the definition of a chiral carbon: A chiral carbon is a carbon atom that is bonded to four different groups or atoms. This lack of symmetry makes the carbon atom chiral.
Examine the molecular structure of each molecule provided in Problem 20.31. Look for carbon atoms that are bonded to four distinct groups or atoms.
For each carbon atom in the molecule, check if it satisfies the criteria for chirality. Ensure that no two substituents attached to the carbon are identical.
Count the number of chiral carbons in each molecule by systematically analyzing each carbon atom and applying the chirality rule.
Repeat the process for all molecules in Problem 20.31, ensuring accuracy in identifying and counting the chiral carbons in each structure.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Chirality
Chirality refers to the geometric property of a molecule that makes it non-superimposable on its mirror image. A chiral molecule typically has at least one carbon atom bonded to four different substituents, resulting in two distinct enantiomers. Understanding chirality is crucial for determining the optical activity and biological interactions of molecules.
Recommended video:
Guided course

Chirality Example 1
Chiral Carbon Atom
A chiral carbon atom, also known as a stereocenter, is a carbon atom that is bonded to four different groups or atoms. The presence of a chiral carbon in a molecule indicates that the molecule can exist in two enantiomeric forms, which can have different chemical and physical properties. Identifying chiral carbons is essential for analyzing the stereochemistry of organic compounds.
Recommended video:
Guided course

Chirality Concept 1
Stereochemistry
Stereochemistry is the branch of chemistry that deals with the spatial arrangement of atoms in molecules and the impact of this arrangement on their chemical behavior. It encompasses concepts such as chirality, enantiomers, and diastereomers. A solid understanding of stereochemistry is vital for predicting the reactivity and interactions of chiral molecules in various chemical contexts.
Recommended video:
Guided course

D vs L Enantiomers Concept 1
Related Practice
Textbook Question
767
views
Textbook Question
Classify the four carbohydrates (a)–(d) by indicating the nature of the carbonyl group and the number of carbon atoms present. For example, glucose is an aldohexose.
c.
675
views
Textbook Question
Classify the four carbohydrates (a)–(d) by indicating the nature of the carbonyl group and the number of carbon atoms present. For example, glucose is an aldohexose.
d.
931
views
Textbook Question
Draw the open-chain structure of a 4-carbon deoxy sugar.
1443
views
Textbook Question
Name four important monosaccharides and tell where each occurs in nature.
1368
views
Textbook Question
Only three stereoisomers are possible for 2,3-dibromo-2, 3-dichlorobutane. Draw them, indicating which pair are enantiomers (optical isomers). Why does the other isomer not have an enantiomer?
707
views
