Consider the trisaccharide A, B, C shown in Problem 20.23.
c. State the numbers of the carbon atoms that form glycosidic linkages between monosaccharide A and monosaccharide B.

 Verified step by step guidance
Verified step by step guidance

Consider the trisaccharide A, B, C shown in Problem 20.23.
c. State the numbers of the carbon atoms that form glycosidic linkages between monosaccharide A and monosaccharide B.
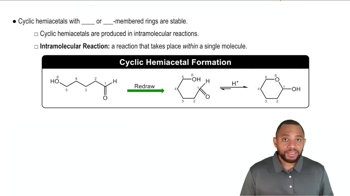
Hydrolysis of both glycosidic bonds in the following trisaccharide A, B, C yields three monosaccharides.
c. Draw the Fischer projections for the three monosaccharides.
Are one or more of the disaccharides maltose, lactose, cellobiose, and sucrose part of the trisaccharide in Problem 20.23? If so, identify which disaccharide and its location. (Hint: Look for an α-1,4 link, β-1,4 link, or 1,2 link, and then determine if the correct monosaccharides are present.)
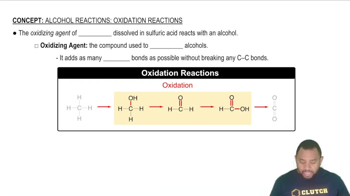
Classify the four carbohydrates (a)–(d) by indicating the nature of the carbonyl group and the number of carbon atoms present. For example, glucose is an aldohexose.
c.
Classify the four carbohydrates (a)–(d) by indicating the nature of the carbonyl group and the number of carbon atoms present. For example, glucose is an aldohexose.
d.
How many chiral carbon atoms are present in each of the molecules shown in Problem 20.31?
a.
b.
c.
d.