Only three stereoisomers are possible for 2,3-dibromo-2, 3-dichlorobutane. Draw them, indicating which pair are enantiomers (optical isomers). Why does the other isomer not have an enantiomer?

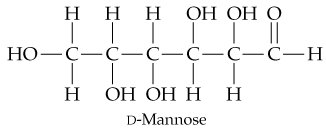
In its open-chain form, D-mannose, an aldohexose found in orange peels, has the structure shown here. Coil mannose around and draw it in the cyclic hemiacetal ⍺ and β forms.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Aldohexose
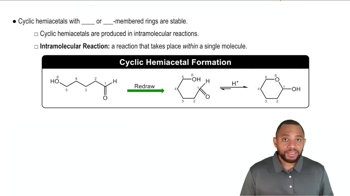
Cyclic Hemiacetal Formation
Anomeric Forms

In Section 15.6, you saw that aldehydes react with reducing agents to yield primary alcohols (RCH=O → RCH2OH). The structures of two D-aldotetroses are shown. One of them can be reduced to yield a chiral product, but the other yields an achiral product. Explain.
Sucrose and D-glucose rotate plane-polarized light to the right; D-fructose rotates light to the left. When sucrose is hydrolyzed, the glucose–fructose mixture rotates light to the left.
b. Why do you think the mixture is called “invert sugar”?
Treatment of D-glucose with a reducing agent yields sorbitol, a substance used as a sugar substitute by people with diabetes. Draw the structure of sorbitol.
Reduction of D-fructose with a reducing agent yields a mixture of D-sorbitol along with a second, isomeric product. What is the structure of the second product?
Treatment of an aldose with an oxidizing agent such as Tollens’ reagent yields a carboxylic acid. Gluconic acid, the product of glucose oxidation, is used as its magnesium salt for the treatment of magnesium deficiency. Draw the structure of gluconic acid.
