Reduction of D-fructose with a reducing agent yields a mixture of D-sorbitol along with a second, isomeric product. What is the structure of the second product?

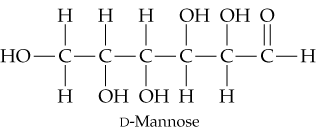
Look at the open-chain form of D-mannose and draw the two glycosidic products that you expect to obtain by reacting D-mannose with methanol.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Glycosidic Bond Formation
D-Mannose Structure
Methanol as a Reactant
Treatment of an aldose with an oxidizing agent such as Tollens’ reagent yields a carboxylic acid. Gluconic acid, the product of glucose oxidation, is used as its magnesium salt for the treatment of magnesium deficiency. Draw the structure of gluconic acid.
Oxidation of the aldehyde group of ribose yields a carboxylic acid. Draw the structure of ribonic acid.
Draw a disaccharide of two cyclic mannose molecules attached by an α-1,4 glycosidic linkage. Explain why the glycosidic products in Problem 20.58 are not reducing sugars, but the product in this problem is a reducing sugar.
Lactose and maltose are reducing disaccharides, but sucrose is a nonreducing disaccharide. Explain.
Amylose (a form of starch) and cellulose are both polymers of glucose. What is the main structural difference between them? What roles do these two polymers have in nature?
