Treatment of an aldose with an oxidizing agent such as Tollens’ reagent yields a carboxylic acid. Gluconic acid, the product of glucose oxidation, is used as its magnesium salt for the treatment of magnesium deficiency. Draw the structure of gluconic acid.
Ch.20 Carbohydrates

Chapter 20, Problem 59
Draw a disaccharide of two cyclic mannose molecules attached by an α-1,4 glycosidic linkage. Explain why the glycosidic products in Problem 20.58 are not reducing sugars, but the product in this problem is a reducing sugar.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the structure of mannose. Mannose is a hexose sugar with the molecular formula C₆H₁₂O₆. In its cyclic form, it exists as a six-membered ring (pyranose) with an anomeric carbon at position 1. The α-anomer has the hydroxyl group on the anomeric carbon pointing down relative to the ring plane.
Step 2: Draw two cyclic mannose molecules. Represent each mannose molecule in its pyranose form, ensuring that the anomeric carbon (C1) is clearly labeled. For the first mannose molecule, use the α-anomer configuration.
Step 3: Form the α-1,4 glycosidic linkage. Connect the anomeric carbon (C1) of the first mannose molecule to the hydroxyl group on carbon 4 (C4) of the second mannose molecule. This bond is called an α-1,4 glycosidic linkage because the linkage involves the α-anomer of the first mannose and the C4 position of the second mannose.
Step 4: Explain why the product is a reducing sugar. A reducing sugar has a free anomeric carbon that can open to form an aldehyde group. In this disaccharide, the second mannose molecule retains its free anomeric carbon (C1), which is not involved in the glycosidic bond. This allows the second mannose to act as a reducing sugar.
Step 5: Contrast with Problem 20.58. In Problem 20.58, the glycosidic products are not reducing sugars because all anomeric carbons are involved in glycosidic bonds, leaving no free anomeric carbon to open and form an aldehyde group. This structural difference explains why the disaccharide in this problem is a reducing sugar, while the products in Problem 20.58 are not.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Disaccharides
Disaccharides are carbohydrates formed by the condensation of two monosaccharides, resulting in a glycosidic bond. In this case, the disaccharide consists of two cyclic mannose molecules linked by an α-1,4 glycosidic bond. This structure is crucial for understanding how sugars can vary in their properties, including their ability to act as reducing or non-reducing sugars.
Recommended video:
Guided course

Types of Disaccharides Concept 1
Glycosidic Linkage
A glycosidic linkage is a covalent bond that connects a carbohydrate molecule to another group, which can be another carbohydrate. The type of glycosidic bond (e.g., α or β) influences the sugar's reactivity and properties. In this context, the α-1,4 glycosidic linkage allows the disaccharide to retain a free anomeric carbon, which is essential for its classification as a reducing sugar.
Recommended video:
Guided course
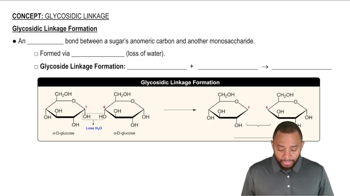
Glycosidic Linkage Formation Concept 1
Reducing Sugars
Reducing sugars are carbohydrates that can donate electrons to other molecules, typically due to the presence of a free aldehyde or ketone group. In the case of the disaccharide formed from mannose, the free anomeric carbon allows it to act as a reducing sugar. In contrast, glycosidic products that do not have a free anomeric carbon are classified as non-reducing sugars, as they cannot participate in redox reactions.
Recommended video:
Guided course

Ketoses as Reducing Sugars Concept 2
Related Practice
Textbook Question
619
views
Textbook Question
Oxidation of the aldehyde group of ribose yields a carboxylic acid. Draw the structure of ribonic acid.
637
views
Textbook Question
Look at the open-chain form of D-mannose and draw the two glycosidic products that you expect to obtain by reacting D-mannose with methanol.
1403
views
Textbook Question
Lactose and maltose are reducing disaccharides, but sucrose is a nonreducing disaccharide. Explain.
944
views
Textbook Question
Amylose (a form of starch) and cellulose are both polymers of glucose. What is the main structural difference between them? What roles do these two polymers have in nature?
571
views
Textbook Question
How are amylose and amylopectin similar to each other, and how are they different from each other?
588
views
