Name the following pathways:
c. Pathway for synthesis of glucose from lactate

 Verified step by step guidance
Verified step by step guidance
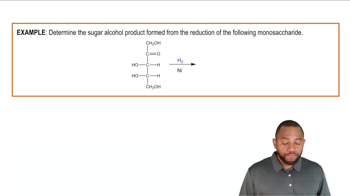

Name the following pathways:
c. Pathway for synthesis of glucose from lactate
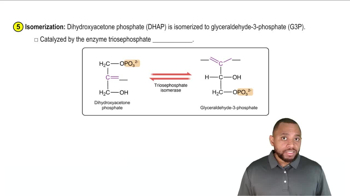
Identify each step in glycolysis that is an isomerization.
Use curved arrows (like those in Figure 22.3) to write an equation for the conversion of fructose to fructose 6-phosphate by ATP. At what step does fructose 6-phosphate enter glycolysis?
In alcoholic fermentation, each mole of pyruvate is converted to one mole of carbon dioxide and one mole of ethanol. In the process, about 50 kcal/mol (209 kJ/mol) of energy is produced. Under the most favorable conditions, more than one-half of this energy is stored as ATP.
a. What happens to the remaining energy produced in alcoholic fermentation?
Pyruvate has three different fates. What are the three different molecules pyruvate is converted into? What conditions exist for the formation of each product?