Textbook Question
Identify each step in glycolysis that is an isomerization.
1525
views

 Verified step by step guidance
Verified step by step guidance

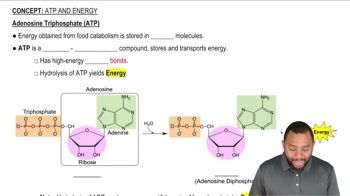

Identify each step in glycolysis that is an isomerization.
In Figure 22.3, compare the starting compound (glucose) and the final product (pyruvate).
a. Which is oxidized to a greater extent?
Use curved arrows (like those in Figure 22.3) to write an equation for the conversion of fructose to fructose 6-phosphate by ATP. At what step does fructose 6-phosphate enter glycolysis?
Pyruvate has three different fates. What are the three different molecules pyruvate is converted into? What conditions exist for the formation of each product?
Glycolysis of one molecule of glucose produces 8 ATP molecules. How many ATP molecules are produced from glycolysis of 10 glucose molecules?
What two types of reactions convert glycerol to dihydroxyacetone phosphate?