Textbook Question
Draw the condensed structural or line-angle formula for the amide formed in each of the following reactions:
c.
51
views

 Verified step by step guidance
Verified step by step guidance

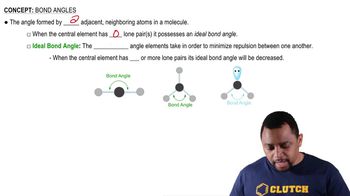

Draw the condensed structural or line-angle formula for the amide formed in each of the following reactions:
c.
Draw the condensed structural or line-angle formula for the amide formed in each of the following reactions:
c.
Write the IUPAC and common names, if any, for each of the following amides:
c.
Draw the condensed structural formula for a and b and the line-angle formula for c and d:
a. heptanamide
Draw the condensed structural formula for a and b and the line-angle formula for c and d:
c. 3-methylbutyramide
Draw the condensed structural or line-angle formulas for the products from the hydrolysis of each of the following amides with HCl:
b.