Indicate whether aqueous solutions of each of the following solutes contain only ions, only molecules, or mostly molecules and a few ions: c. fructose, C6H12O6, a nonelectrolyte
Ch.9 Solutions

Timberlake14thChemistry: An Introduction to General, Organic, and Biological ChemistryISBN: 9781292472249Not the one you use?Change textbook
Chapter 9, Problem 13b
Classify the solute represented in each of the following equations as a strong, weak, or nonelectrolyte:
b. NH3(g) + H2O(l) ⇌ NH4+(aq) + OH–(aq)
 Verified step by step guidance
Verified step by step guidance1
Identify the solute in the given equation. In this case, the solute is NH₃ (ammonia).
Examine the reaction provided: NH₃(g) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq). Notice the double arrow (⇌), which indicates that the reaction is reversible and does not go to completion.
Understand the behavior of NH₃ in water. Ammonia reacts with water to form NH₄⁺ (ammonium ion) and OH⁻ (hydroxide ion), but only a small fraction of NH₃ molecules ionize. This partial ionization is characteristic of a weak electrolyte.
Recall the definition of a weak electrolyte: A weak electrolyte partially ionizes in solution, producing a small number of ions while most of the solute remains in its molecular form.
Classify NH₃ as a weak electrolyte based on its partial ionization in water and the reversible nature of the reaction.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electrolytes
Electrolytes are substances that dissociate into ions when dissolved in water, allowing the solution to conduct electricity. They are classified into strong electrolytes, which completely dissociate into ions, weak electrolytes, which partially dissociate, and nonelectrolytes, which do not dissociate at all. Understanding this classification is crucial for analyzing chemical reactions in aqueous solutions.
Recommended video:
Guided course
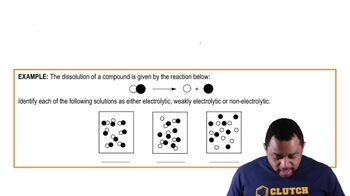
Electrolytes (Simplified) Example 3
Strong vs. Weak Electrolytes
Strong electrolytes, such as sodium chloride, fully ionize in solution, resulting in a high concentration of ions. In contrast, weak electrolytes, like ammonia (NH₃), only partially ionize, leading to a lower concentration of ions. This distinction affects the conductivity of the solution and is essential for classifying solutes in chemical equations.
Recommended video:
Guided course
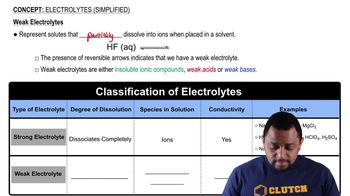
Electrolytes (Simplified) Concept 2
Ammonia in Water
When ammonia (NH₃) is dissolved in water, it acts as a weak base, partially reacting with water to form ammonium ions (NH₄⁺) and hydroxide ions (OH⁻). This equilibrium reaction indicates that ammonia is a weak electrolyte, as it does not fully dissociate into ions. Recognizing this behavior is key to classifying ammonia in the context of electrolyte strength.
Recommended video:
Guided course
Tollens' Test Concept 2
Related Practice
Textbook Question
1733
views
Textbook Question
Indicate whether aqueous solutions of each of the following solutes contain only ions, only molecules, or mostly molecules and a few ions:
b. NaBr, a strong electrolyte
2020
views
Textbook Question
Classify the solute represented in each of the following equations as a strong, weak, or nonelectrolyte:
a.
1824
views
Textbook Question
Calculate the number of equivalents in each of the following:
d. 3 moles of CO32–
1144
views
Textbook Question
Calculate the number of equivalents in each of the following:
d. 2 moles of Fe3+
1372
views
Textbook Question
An intravenous saline solution contains 154 mEq/L each of Na+ and Cl–. How many moles each of Na+ and Cl– are in 1.00 L of the saline solution?
1423
views
