Textbook Question
Determine the products of each reaction. b.
220
views

 Verified step by step guidance
Verified step by step guidance


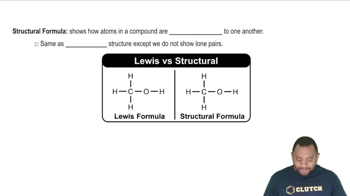
Determine the products of each reaction. b.
Determine the products of each reaction. c.
Determine the products of each reaction. d.
Identify the two compounds that display stereoisomerism and draw their structures. a. 3-methyl-3-pentanol b. 2-methyl-2-pentanol c. 3-methyl-2-pentanol d. 2-methyl-3-pentanol e. 2,4-dimethyl-3-pentanol
There are eight structures with the formula C3H7NO in which the O is part of a carbonyl group. Draw the structures and identify the functional groups in each.