Textbook Question
Write orbital diagrams for each of these ions.Determine if the ion is diamagnetic or paramagnetic. a. V5+ b. Cr3+ c. Ni2+ d. Fe3+
641
views

 Verified step by step guidance
Verified step by step guidance
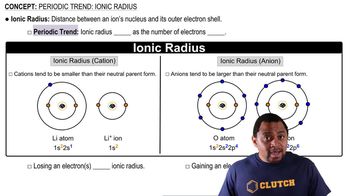


Write orbital diagrams for each of these ions.Determine if the ion is diamagnetic or paramagnetic. a. V5+ b. Cr3+ c. Ni2+ d. Fe3+
Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd2+ b. Au+ c. Mo3+ d. Zr2+
Which is the larger species in each pair? a. Li or Li+ b. Cs- or Cs+ c. Cr- or Cr3+ d. O or O2-
Arrange this isoelectronic series in order of increasing atomic radius: Se2- , Sr2+ , Rb+ , Br- .
Choose the element with the higher first ionization energy from each pair. a. Br or Bi