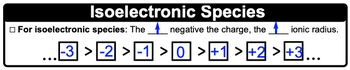
Understanding ionic radius is crucial in the study of chemistry, particularly when examining the behavior of ions. The ionic radius is defined as the distance from the nucleus of an ion to its outermost electron shell. Unlike many periodic trends that can be inferred from the periodic table, the ionic radius trend is determined by the number of electrons present in the ion.
As a general rule, the ionic radius increases with the addition of electrons. This means that anions, which are negatively charged ions formed by gaining electrons, tend to have a larger radius than their neutral parent atoms. For example, when oxygen (which has the electron configuration 1s2 2s2 2p4) gains two electrons to become an oxide ion, its outer shell now contains eight electrons. This increase in electron count results in a larger ionic radius compared to the neutral oxygen atom.
Conversely, cations, or positively charged ions formed by losing electrons, exhibit a smaller ionic radius than their neutral counterparts. Taking lithium as an example, the lithium atom has the electron configuration 1s2 2s1. When it loses one electron to form a lithium ion, it now has only one electron shell, leading to a decrease in size. Thus, the trend indicates that losing electrons results in a smaller ionic radius.
In summary, the ionic radius is influenced by the charge of the ion: cations are smaller than their neutral forms due to the loss of electrons, while anions are larger due to the gain of electrons. Therefore, when assessing ionic radius, focus on the total number of electrons in the ion rather than relying on periodic table trends.