Textbook Question
Draw structures of the carboxylic acids and alcohols you would use to prepare each ester in Problem 17.54.
a.
b.
c. Cyclohexyl acetate
d. Phenyl-o-hydroxybenzoate
732
views

 Verified step by step guidance
Verified step by step guidance


Draw structures of the carboxylic acids and alcohols you would use to prepare each ester in Problem 17.54.
a.
b.
c. Cyclohexyl acetate
d. Phenyl-o-hydroxybenzoate
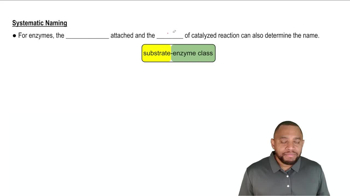
Give systematic names for the following structures and structures for the names:
c. N-Ethyl-N-methylbenzamide
Give systematic names for the following structures and structures for the names:
a. 3-Methylpentanamide
When both the carboxylic acid and the amine are in the same molecule, amide formation produces lactams. A lactam is a cyclic amide, where the amide group is part of the ring. Draw the structure of the product(s) obtained from acid hydrolysis of these lactams.
a.