If glycolysis occurs in the cytoplasm and the citric acid cycle occurs in the mitochondrial matrix, how do the products of glycolysis get inside the mitochondrial matrix?
Ch.12 Food as Fuel An Overview of Metabolism

Frost4th EditionGeneral, Organic and Biological ChemistryISBN: 9780134988696Not the one you use?Change textbook
Chapter 8, Problem 74
Which of the reactions given in Problems 12.72 represent isomerizations where the reactants and products are structural isomers?
a. glucose to glucose-6-phosphate
b. glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate
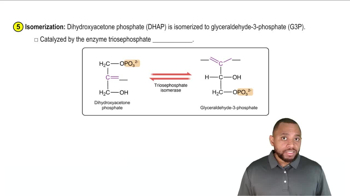
c. dihydroxyacetone phosphate to glyceraldehyde-3-phosphate
 Verified step by step guidance
Verified step by step guidance1
Understand the concept of isomerization: Isomerization is a chemical reaction where a molecule is transformed into another molecule with the same molecular formula but a different structural arrangement. Structural isomers differ in the connectivity of atoms within the molecule.
Review the reactions provided in Problems 12.72. For each reaction, compare the molecular formula of the reactants and products to ensure they are identical. This confirms that the reactants and products are isomers.
Examine the structural arrangement of the reactants and products. Check if the connectivity of atoms changes between the reactants and products, which is a key characteristic of structural isomers.
Identify any reactions where the molecular formula remains the same and the connectivity of atoms changes. These reactions represent isomerizations involving structural isomers.
List the reactions that meet the criteria for isomerization. Ensure that you clearly distinguish these from other types of reactions, such as rearrangements that do not involve structural isomers.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Isomerization
Isomerization is a chemical reaction in which a molecule is transformed into another molecule that has the same atoms but a different arrangement. This process can lead to structural isomers, which differ in the connectivity of their atoms, or stereoisomers, which differ in the spatial arrangement of atoms. Understanding isomerization is crucial for identifying reactions that produce structural isomers.
Recommended video:
Guided course
Glycolysis Concept 5
Structural Isomers
Structural isomers are compounds that share the same molecular formula but have different structural formulas, meaning the connectivity of atoms varies. This can result in different physical and chemical properties. Recognizing structural isomers is essential for analyzing reactions, as it helps determine whether a reaction leads to a new compound with distinct characteristics.
Recommended video:
Guided course

Isomers Concept 1
Reaction Mechanism
A reaction mechanism is a detailed description of the steps involved in a chemical reaction, including the formation and breaking of bonds. Understanding the mechanism allows chemists to predict the products of a reaction and identify whether isomerization occurs. Analyzing the mechanism is key to determining if the reactants and products are structural isomers.
Recommended video:
Guided course

Alcohol Reactions: Dehydration Reactions Concept 1
Related Practice
Textbook Question
485
views
Textbook Question
Which of the following reactions in glycolysis produce ATP or NADH?
a. 1,3-bisphosphoglycerate to 3-phosphoglycerate
1114
views
Textbook Question
Which of the following reactions in glycolysis produce ATP or NADH?
a. glucose to glucose-6-phosphate
1335
views
Textbook Question
Refer to the diagram of the citric acid cycle in Figure 12.11 to answer each of the following:
d. Name the reactions where secondary alcohols are oxidized to ketones.
455
views
Textbook Question
Refer to the diagram of the citric acid cycle in Figure 12.11 to answer each of the following:
c. Name the reaction that is coupled to GTP formation.
515
views
Textbook Question
If there are no reactions in the citric acid cycle that use oxygen, O2, why does the cycle operate only in aerobic conditions?
697
views
