Textbook Question
Classify each of the following monosaccharides by the type of carbonyl group and the number of carbons (for example, a monosaccharide with an aldehyde and three carbons is an aldotriose).
(a)
634
views

 Verified step by step guidance
Verified step by step guidance



Classify each of the following monosaccharides by the type of carbonyl group and the number of carbons (for example, a monosaccharide with an aldehyde and three carbons is an aldotriose).
(a)
Identify the following monosaccharides as the D- or the L-isomer:
(a)
Draw the Fischer projection for the enantiomer (mirror image) of each of the following:
(a)
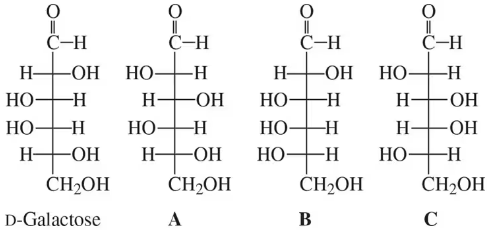
Use the structure of D-galactose in Problem 6.15 to answer the following:
(a) Draw the Fischer projection of the carbon 3 epimer.
Use the structure of d-galactose in Problem 6.15 to answer the following:
(b) Draw the Fischer projection of L-galactose.
Identify the monosaccharide that fits each of the following descriptions:
(a) also referred to as dextrose