What is meant by the following terms as they apply to protein structure, and what bonds or molecular interactions stabilize that level of structure?
d. Quaternary structure

 Verified step by step guidance
Verified step by step guidance
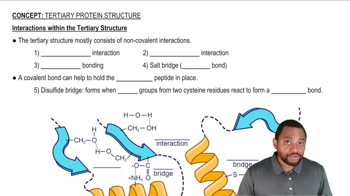

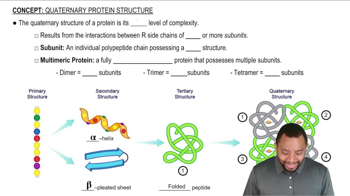
What is meant by the following terms as they apply to protein structure, and what bonds or molecular interactions stabilize that level of structure?
d. Quaternary structure
What level of protein structure is determined by the following:
a. Peptide bonds between amino acids?
What level of protein structure is determined by the following:
b. Hydrogen bonds between backbone carbonyl oxygen atoms and hydrogen atoms attached to backbone nitrogen atoms?
How do the following interactions help to stabilize the tertiary and quaternary structure of a protein? Give an example of a pair of amino acids that could give rise to each interaction.
b. Disulfide bonds
Give an example of a protein that has quaternary structure. How many polypeptide chains are present in this protein?
Explain how a protein is denatured by the following:
a. Heat